Phase I/II Trial of Perioperative Avelumab in Combination With Chemoradiation in the Treatment of Stage II/III Resectable Esophageal and Gastroesophageal Junction Cancer
- PMID: 39757733
- PMCID: PMC12186108
- DOI: 10.1002/jso.28070
Phase I/II Trial of Perioperative Avelumab in Combination With Chemoradiation in the Treatment of Stage II/III Resectable Esophageal and Gastroesophageal Junction Cancer
Abstract
Background and objectives: Standard treatment of patients with stage II/III esophageal or gastroesophageal junction (E/GEJ) cancer involves neoadjuvant chemoradiation (nCRT), resection, and immunotherapy. Our trial evaluated the addition of perioperative avelumab to standard treatments.
Methods: Patients with resectable E/GEJ cancers received avelumab with nCRT and adjuvant avelumab after resection. Primary endpoints for phase I and II portions were safety and pathologic complete response (pCR) rate, respectively. Secondary endpoints included recurrence-free survival (RFS), surgical complication prevalence, and R0 resection rate.
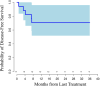
Results: Twenty-two patients enrolled in the study. Median follow-up during data cutoff was 23.9 months. There were no dose-limiting toxicities during the run-in phase. Nineteen patients (86.4%) underwent resection with R0 resection rate of 78.9% and with pCR rate of 26%. Most common treatment-related adverse events (TRAE) were cytopenias from chemoradiation. Aside from one grade ≥ 3 avelumab-related hypersensitivity, no grade ≥ 3 avelumab TRAEs were seen. Median RFS was not reached, and 1-year RFS and overall survival were 71% and 81%, respectively. The study was terminated before full planned accrual due to standard practice change based on the CheckMate 577 trial.
Conclusions: The addition of perioperative avelumab to nCRT was tolerable and demonstrated promising outcomes.
Keywords: chemoradiation; esophageal cancer; gastroesophageal adenocarcinoma; immunotherapy.
© 2025 The Author(s). Journal of Surgical Oncology published by Wiley Periodicals LLC.
Figures
References
-
- Key Statistics for Esophageal Cancer . 2024, https://www.cancer.org/cancer/types/esophagus-cancer/about/key-statistic....
-
- Eyck B. M., van Lanschot J. J. B., Hulshof M. C. C. M., et al., “Ten‐Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial,” Journal of Clinical Oncology 39, no. 18 (2021): 1995–2004. - PubMed
-
- Kelly R. J., Ajani J. A., Kuzdzal J., et al., “Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer,” New England Journal of Medicine 384, no. 13 (2021): 1191–1203. - PubMed
-
- Blum Murphy M., Xiao L., Patel V. R., et al., “Pathological Complete Response in Patients With Esophageal Cancer After the Trimodality Approach: The Association With Baseline Variables and Survival‐The University of Texas MD Anderson Cancer Center Experience,” Cancer 123, no. 21 (2017): 4106–4113. - PubMed
-
- van Hagen P., Hulshof M. C. C. M., van Lanschot J. J. B., et al., “Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer,” New England Journal of Medicine 366, no. 22 (2012): 2074–2084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical