Expression of osteogenic proteins in kidneys of cats with nephrocalcinosis
- PMID: 39757788
- PMCID: PMC11702495
- DOI: 10.1111/jvim.17278
Expression of osteogenic proteins in kidneys of cats with nephrocalcinosis
Abstract
Background: Nephrocalcinosis is a common pathological finding in cats with chronic kidney disease and nephrolithiasis. Understanding its pathogenesis may identify future therapeutic targets.
Hypothesis: Nephrocalcinosis is associated with expression of an osteogenic phenotype.
Animals: Kidneys with medullary mineralization were obtained from 18 cats (10 with and 8 without nephroliths) undergoing necropsy.
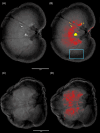
Methods: Cross-sectional study. Microradiography and histopathology (modified von Kossa stain) were used to confirm parenchymal mineralization. Immunohistochemistry for 5 osteogenic markers was performed to determine their co-localization with nephrocalcinosis. The proportion of kidneys with stronger immunointensity in mineralized versus non-mineralized regions was analyzed using 1-tailed sign tests. The proportion of kidneys with co-localization of nephrocalcinosis and each marker was compared between kidneys with and without nephroliths using Fisher's exact tests.
Results: Nephrocalcinosis co-localized with osteopontin immunoreactivity in all 18 cats (100%) and with osteocalcin in 12 cats (67%). Both osteogenic markers had stronger immunointensity in mineralized regions compared with non-mineralized regions. Limited co-localization was observed with other markers: bone morphogenic protein-2 in 2 kidneys (both with nephroliths) and tissue non-specific alkaline phosphatase in 1 kidney (without nephroliths); runt-related transcription factor-2 was undetected. No statistically significant differences were found in the co-localization of nephrocalcinosis with osteogenic proteins between kidneys with and without nephroliths.
Conclusions and clinical importance: Expression of osteogenic proteins in areas of nephrocalcinosis indicates that nephrocalcinosis is associated with the development of an osteogenic phenotype. Targeting these processes could offer a novel approach to prevent nephrolithiasis at its origin.
Keywords: chronic kidney disease; immunohistochemistry; mineralization; nephroliths; radiography.
© 2025 The Author(s). Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures


Similar articles
-
Detection of nephrocalcinosis using ultrasonography, micro-computed tomography, and histopathology in cats.J Vet Intern Med. 2024 May-Jun;38(3):1553-1562. doi: 10.1111/jvim.17011. Epub 2024 Feb 13. J Vet Intern Med. 2024. PMID: 38348812 Free PMC article.
-
Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells.Stem Cell Res Ther. 2017 Feb 21;8(1):39. doi: 10.1186/s13287-017-0497-2. Stem Cell Res Ther. 2017. PMID: 28222767 Free PMC article.
-
Risk factors and implications associated with renal mineralization in chronic kidney disease in cats.J Vet Intern Med. 2022 Mar;36(2):634-646. doi: 10.1111/jvim.16363. Epub 2022 Jan 19. J Vet Intern Med. 2022. PMID: 35043997 Free PMC article.
-
Nephroliths and ureteroliths: a new stone age.N Z Vet J. 2013 Jul;61(4):212-6. doi: 10.1080/00480169.2013.775691. Epub 2013 Mar 14. N Z Vet J. 2013. PMID: 23484823 Review.
-
Nephrocalcinosis caused by hyperparathyroidism in progression of renal failure: treatment with calcitriol.Semin Vet Med Surg Small Anim. 1992 Aug;7(3):202-20. Semin Vet Med Surg Small Anim. 1992. PMID: 1410853 Review.
References
-
- Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26:275‐281. - PubMed
-
- Lulich J. (2016). Microanatomy of Feline Nephrolithiasis. Proceedings of the American College of Veterinary Internal Medicine (ACVIM) Forum, June 8‐11, Denver, CO, USA. American College of Veterinary Internal Medicine. ISBN: 9781510825406.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

