A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of a Nutraceutical Supplement With Standardized Botanicals in Males With Thinning Hair
- PMID: 39757794
- PMCID: PMC11701407
- DOI: 10.1111/jocd.16778
A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of a Nutraceutical Supplement With Standardized Botanicals in Males With Thinning Hair
Abstract
Background: Hair thinning in men is a prevalent issue for which treatment oftentimes consists of a multi-modal approach. Targeting key root causes of hair thinning, such as hormones, stress, and metabolism through vitamins, minerals, and botanicals, has been shown to be effective in improving hair growth and quality in women. This approach could also be effective in improving hair growth and quality in men with thinning hair.
Aims: This study investigates the safety and efficacy of a nutraceutical in improving hair growth and quality in men with thinning hair.
Methods: This was a 6-month multi-center, double-blind, randomized, placebo-controlled trial. Men aged 21-61 years old with confirmed hair thinning were included. Subjects were randomized to receive the oral supplement or placebo. The study end points included changes in blinded Global Investigator Ratings for hair growth and quality, the Men's Hair Growth Questionnaire (MHGQ), and changes in the Arizona Sexual Experience Scale (ASEX) in the active group compared to baseline and placebo.
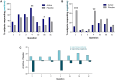
Results: Daily intake of the nutraceutical resulted in significantly more subjects in the active group compared to the placebo rated as improved for hair growth and quality in the blinded investigator global assessments. Overall changes and responses to the MHGQ also corroborated blinded investigator ratings. There were no trends in either treatment group for changes in sexual function on the ASEX questionnaire, and the supplement was well tolerated.
Conclusions: Ingestion of a nutraceutical targeting key root causes of male hair thinning significantly improves hair growth and quality in men with no changes in sexual function.
Keywords: MPHL; botanicals; hair thinning; male pattern hair loss; nutraceutical; supplement.
© 2025 Nutraceutical Wellness, Inc. Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Neal Bhatia is an advisor for Nutrafol and has received research grants from Nutraceutical Wellness Inc. Dr. Glynis Ablon has previously received research grants from Nutraceutical Wellness Inc. Dr. Patricia K. Farris is an advisor for Nutrafol and Nutraceutical Wellness Inc. Drs. Adina Hazan and Isabelle Raymond are employees of Nutraceutical Wellness Inc.
Figures
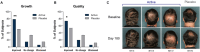
References
-
- American Hair Loss Association , “Men's Hair Loss,” 2024, accessed August 27, 2024, https://www.americanhairloss.org/mens‐hair‐loss/.
-
- Farris P. K., Rogers N., McMichael A., and Kogan S., “A Novel Multi‐Targeting Approach to Treating Hair Loss, Using Standardized Nutraceuticals,” Journal of Drugs in Dermatology 16, no. 11 (2017): s141–s148. - PubMed
-
- Stephens T. J., Berkowitz S., Marshall T., Kogan S., and Raymond I., “A Prospective Six‐Month Single‐Blind Study Evaluating Changes in Hair Growth and Quality Using a Nutraceutical Supplement in Men and Women of Diverse Ethnicities,” Journal of Clinical and Aesthetic Dermatology 15, no. 1 (2022): 21–26. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

