Enhancing Chitin Production as a Fermentation Byproduct through a Genetic Toolbox That Activates the Cell Wall Integrity Response
- PMID: 39757911
- PMCID: PMC11744920
- DOI: 10.1021/acssynbio.4c00436
Enhancing Chitin Production as a Fermentation Byproduct through a Genetic Toolbox That Activates the Cell Wall Integrity Response
Abstract
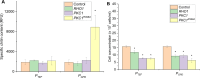
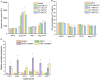
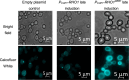
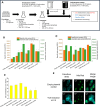
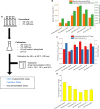
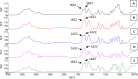
Often, the value of the whole biomass from fermentation processes is not exploited, as commercial interests are focused on the main product that is typically either accumulated within cells or secreted into the medium. One underutilized fraction of yeast cells is the cell wall that contains valuable polysaccharides, such as chitin, known for its biocompatibility and biodegradability, which are thought of as valuable properties in diverse industries. Therefore, the valorization of waste biomass from fermentation to coproduce chitin could significantly improve the overall profitability and sustainability of biomanufacturing processes. Previous studies revealed that environmental stresses trigger the cell wall integrity (CWI) response, leading to an increased level of chitin synthesis as a protective measure. In this study, we evaluated the use of the key regulatory genes of the CWI response, RHO1 and PKC1, and their mutant forms RHO1Q68H and PKC1R398A, to design a genetic switch that provides control over the CWI response to maximize the chitin content in the cell wall. The generated genetic control elements were introduced into different yeast strains, among others, for the coproduction of chitin with either storage lipids or recombinant proteins. Overall, we successfully increased the chitin content in the yeast cell wall up to five times with our optimized setup. Furthermore, similar improvements in chitin production were seen when coproducing chitin with either storage lipids or a secreted acid phosphatase. Our results successfully demonstrated the potential of maximizing the chitin content in the cell wall fraction while producing other intra- or extracellular compounds, showcasing a promising approach for enhancing the efficiency and sustainability of fermentation processes. Moreover, the chitin produced in the cell wall is indistinguishable from the chitin isolated from crustaceans.
Keywords: PKC1; RHO1; Saccharomyces cerevisiae; cell wall integrity pathway; chitin; coproduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Crognale S.; Russo C.; Petruccioli M.; D’Annibale A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76.10.3390/fermentation8020076. - DOI
-
- Coma V.; Bartkowiak A.. Potential of Chitosans in the Development of Edible Food Packaging. In Chitin and chitosan: Properties and applications; Wiley Online Library, 2019, pp. 349–369.
-
- Dave U.; Somanader E.; Baharlouei P.; Pham L.; Rahman M. A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173.10.3390/jmse9111173. - DOI
-
- Samoila P.; Humelnicu A. C.; Ignat M.; Cojocaru C.; Harabagiu V. Chitin and Chitosan for Water Purification. Chitin Chitosan 2019, 429–460. 10.1002/9781119450467.ch17. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

