Therapeutic effects of chlorogenic acid on allergic rhinitis through TLR4/MAPK/NF-κB pathway modulation
- PMID: 39757930
- PMCID: PMC12097400
- DOI: 10.17305/bb.2024.11582
Therapeutic effects of chlorogenic acid on allergic rhinitis through TLR4/MAPK/NF-κB pathway modulation
Abstract
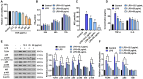
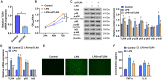
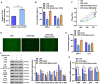
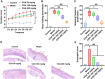
Chlorogenic acid (CGA) exhibits promising anti-inflammatory properties, making it a potential therapeutic agent for inflammatory conditions and allergic rhinitis (AR). This study aimed to evaluate the therapeutic effects of CGA on inflammation in RAW264.7 macrophage cells and on AR in mice. RAW264.7 cells were treated with lipopolysaccharide (LPS) to induce inflammation and cultured with varying concentrations of CGA, a Tlr4-silenced gene (shTlr4) transfection, and the MAPK/NF-κB pathway activator diprovocim. Cell viability was assessed using the CCK8 assay, while levels of nitric oxide (NO), TNF-α, and IL-6 were measured by Griess colorimetry, immunofluorescence, and ELISA. Expression and phosphorylation levels of the MAPK/NF-κB pathway were evaluated using qPCR and western blotting. Additionally, ovalbumin (OVA)-induced AR mice received different doses of CGA, and Toll-like receptor-4 (Tlr4) overexpression was induced. In vitro, CGA treatment significantly reduced LPS-induced cell activity, NO, TNF-α, and IL-6 secretion, and downregulated Tlr4, p-p38, p-p65, and p-IκB expression. Tlr4 inhibition suppressed cell activity and inflammation by blocking MAPK/NF-κB pathway activation. Conversely, Tlr4 overexpression counteracted the effects of CGA, increasing cell activity and inflammatory factor concentration. In OVA-induced AR mice, CGA effectively alleviated allergic symptoms, reduced inflammatory factor secretion, and inhibited TLR4/MAPK/NF-κB pathway activity. These findings suggest CGA's potential as an anti-inflammatory agent in RAW264.7 cells and AR models through modulation of the TLR4/MAPK/NF-κB pathway.
Conflict of interest statement
Conflicts of interest: Authors declare no conflicts of interest.
Figures
References
-
- Miraglia Del Giudice M, Allegorico A, Marseglia GL, Martelli A, Calvani M, Cardinale F, et al. Allergic rhinoconjunctivitis. Acta Biomed. 2020;91(11):e2020007. https://doi.org/10.23750/abm.v91i11-S.10310. - PMC - PubMed
-
- Ding MR, Liang QL, Xu HG, Li XD, Zhang K, Wei ZJ, et al. Smart peptide defense web in situ connects for continuous interception of IgE against allergic rhinitis. ACS Appl Mater Interfaces. 2022;14(26):29639–49. https://doi.org/10.1021/acsami.2c07092. - PubMed
-
- Fonseca J, Taveira-Gomes T, Pereira AM, Branco-Ferreira M, Carreiro-Martins P, Alves-Correia M, et al. ARIA 2019: um percurso assistencial integrado para a rinite alérgica em Portugal [ARIA 2019: an integrated care pathway for allergic rhinitis in Portugal] Acta Med Port. 2021;34(2):144–57. https://doi.org/10.20344/amp.13777. - PubMed
-
- Meng Y, Wang C, Zhang L. Recent developments and highlights in allergic rhinitis. Allergy. 2019;74(12):2320–8. https://doi.org/10.1111/all.14067. - PubMed
-
- Athari SS. Targeting cell signaling in allergic asthma. Signal Transduct Target Ther. 2019;4:45. https://doi.org/10.1038/s41392-019-0079-0. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

