The role of cisplatin in modulating the tumor immune microenvironment and its combination therapy strategies: a new approach to enhance anti-tumor efficacy
- PMID: 39757995
- PMCID: PMC11705547
- DOI: 10.1080/07853890.2024.2447403
The role of cisplatin in modulating the tumor immune microenvironment and its combination therapy strategies: a new approach to enhance anti-tumor efficacy
Abstract
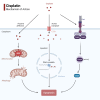
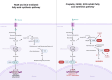
Cisplatin is a platinum-based drug that is frequently used to treat multiple tumors. The anti-tumor effect of cisplatin is closely related to the tumor immune microenvironment (TIME), which includes several immune cell types, such as the tumor-associated macrophages (TAMs), cytotoxic T-lymphocytes (CTLs), dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and natural killer (NK) cells. The interaction between these immune cells can promote tumor survival and chemoresistance, and decrease the efficacy of cisplatin monotherapy. Therefore, various combination treatment strategies have been devised to enhance patient responsiveness to cisplatin therapy. Cisplatin can augment anti-tumor immune responses in combination with immune checkpoint blockers (such as PD-1/PD-L1 or CTLA4 inhibitors), lipid metabolism disruptors (like FASN inhibitors and SCD inhibitors) and nanoparticles (NPs), resulting in better outcomes. Exploring the interaction between cisplatin and the TIME will help identify potential therapeutic targets for improving the treatment outcomes in cancer patients.
Keywords: Cisplatin; combination therapy; immune therapy; lipid metabolism disruptors; tumor immune microenvironment.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
