Young Psoriatic Patients Respond Faster to Dimethyl Fumarate: Age-related Differences in Efficacy and Adverse Events
- PMID: 39758220
- PMCID: PMC11694644
Young Psoriatic Patients Respond Faster to Dimethyl Fumarate: Age-related Differences in Efficacy and Adverse Events
Abstract
Background: Dimethyl fumarate (DMF) is an oral treatment approved by the European Medicines Agency (EMA) to treat moderate-to-severe plaque psoriasis among adult patients.
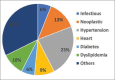
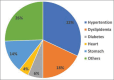
Objectives: This study aims to evaluate sociodemographic, anthropometric, and medical characteristics in patients with psoriasis without previous history of traditional systemic therapy and to observe if the efficacy or AEs of dimethyl fumarate correlate to any of the patients' characteristics.
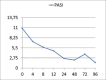
Methods: Ninety-two patients with mild-to-moderate psoriasis were enrolled. Each patient was reviewed at 4, 12, 24 and 36 weeks. The PASI score and any clinical side effects or blood count abnormalities were recorded.
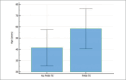
Results: After 4 weeks, a decrease in the median value of PASI index was already noticeable (p<0.001). After 12 weeks of treatment, 43.9% of patients reached PASI-50, 12.3% PASI-75. The patients achieving PASI-75 after 12 weeks of treatment were significantly younger than those who did not. Age, BMI index, gender and gastroprotection used were not significantly related to the occurrence of side effects.
Conclusion: Profiling of patients could be useful in predicting the response to treatment. In our study, younger patients were found to respond better to dimethyl fumarate.
Keywords: adverse events, psoriasis; age; dimethyl fumarate; efficacy.
Copyright © 2024. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Drs. Burlando and Parodi have served as speakers for AbbVie, Amgen, Almirall, Eli Lilly, Janssen, Novartis, and UCB.
Figures
Similar articles
-
Dimethyl Fumarate as Therapeutic Alternative in Moderate-to-Severe Psoriasis: Our Experience.Psoriasis (Auckl). 2022 Jun 29;12:177-185. doi: 10.2147/PTT.S367060. eCollection 2022. Psoriasis (Auckl). 2022. PMID: 35791415 Free PMC article.
-
Dimethyl Fumarate: A Review in Moderate to Severe Plaque Psoriasis.Drugs. 2018 Jan;78(1):123-130. doi: 10.1007/s40265-017-0854-6. Drugs. 2018. PMID: 29236231 Review.
-
Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: Results from a 52-Week Open-Label Phase IV Clinical Trial (DIMESKIN 1).Dermatol Ther (Heidelb). 2023 Jan;13(1):329-345. doi: 10.1007/s13555-022-00863-2. Epub 2022 Dec 1. Dermatol Ther (Heidelb). 2023. PMID: 36456890 Free PMC article.
-
Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus.J Eur Acad Dermatol Venereol. 2018 Oct;32 Suppl 3:3-14. doi: 10.1111/jdv.15218. Epub 2018 Sep 20. J Eur Acad Dermatol Venereol. 2018. PMID: 30238510
-
Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm® - and placebo-controlled trial (BRIDGE).Br J Dermatol. 2017 Mar;176(3):615-623. doi: 10.1111/bjd.14947. Epub 2016 Nov 15. Br J Dermatol. 2017. PMID: 27515097 Clinical Trial.
References
-
- Mrowietz U, Barker J, Boehncke WH et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(Suppl 3):3–14. - PubMed
-
- Carboni I, De Felice C, De Simoni I et al. Fumaric acid esters in the treatment of Psoriasis: an Italian experience. J Dermatolog Treat. 2004;15(1):23–26. - PubMed
-
- Balasubramaniam P, Stevenson O, Berth-Jones J. Fumaric acid esters in severe psoriasis, including experience of use in combination with other systemic modalities. Br J Dermatol. 2004;150(4):741–746. - PubMed
-
- Heelan K, Markham T. Fumaric acid esters as a suitable first-line treatment for severe psoriasis: an Irish experience. Clin Exp Dermatol. 2012;37(7):793–795. - PubMed
-
- Pezzolo E, Cazzaniga S, Di Leo S et al. Efficacy and safety of dimethyl fumarate in comparison with conventional therapy for psoriasis: an Italian real-world clinical experience. J Eur Acad Dermatol Venereol. 2022;36(7):e534–e537. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials