Microfluidic-based electrically driven particle manipulation techniques for biomedical applications
- PMID: 39758908
- PMCID: PMC11697266
- DOI: 10.1039/d4ra05571c
Microfluidic-based electrically driven particle manipulation techniques for biomedical applications
Abstract
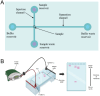
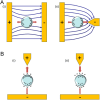
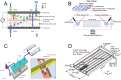
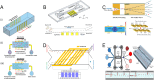
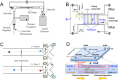
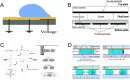
Microfluidic chips exhibit unique advantages in both economy and rapidity, particularly for the separation and detection of biomolecules. In this review, we first introduced the mechanisms of several electrically driven methods, such as electrophoresis, dielectrophoresis, electro-wetting and electro-rotation. We then discussed in detail the application of these methods in nucleic acid analysis, protein manipulation and cell treatment. In addition, we outlined the considerations for material selection, manufacturing processes and structural design of microfluidic chips based on electrically driven mechanisms.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Jiang J. Cui X. Huang Y. Yan D. Wang B. Yang Z. Chen M. Wang J. Zhang Y. Liu G. Advances and Prospects in Integrated Nano-oncology. Nano Biomed. Eng. 2024;16(2):152–187.
-
- Tian Q. Mu Y. Xu Y. Song Q. Yu B. Ma C. Jin W. Jin Q. An integrated microfluidic system for bovine DNA purification and digital PCR detection. Anal. Biochem. 2015;491:55–57. - PubMed
-
- Chen M. Lin S. Zhou C. Cui D. Haick H. Tang N. From conventional to microfluidic: progress in extracellular vesicle separation and individual characterization. Adv. Healthcare Mater. 2023;12(8):2202437. - PubMed
-
- Yatsushiro S. Yamaguchi Y. Yamamura S. Shinohara Y. Baba Y. Kataoka M. Highly sensitive DNA detection with a combination of 2 DNA-intercalating dyes for microchip electrophoresis. J. Pharm. Biomed. Anal. 2011;55(1):202–205. - PubMed
-
- Qian J.-J. Ji H.-Y. Cong H. Wang H.-M. Jin Q.-H. Application of Polydimethylsiloxane/Glass Microchips for Fast Electrophoretic Separation of Serum High-density Lipoprotein Subclasses. Chin. J. Anal. Chem. 2012;40(2):230–235.
Publication types
LinkOut - more resources
Full Text Sources

