Design, synthesis, anticancer activity and molecular docking of quinoline-based dihydrazone derivatives
- PMID: 39758910
- PMCID: PMC11694625
- DOI: 10.1039/d4ra06954d
Design, synthesis, anticancer activity and molecular docking of quinoline-based dihydrazone derivatives
Abstract
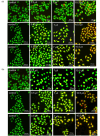
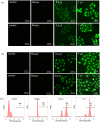
Based on the biologically active heterocycle quinoline, we successfully synthesized a series of quinoline-based dihydrazone derivatives (3a-3d). 1H NMR, 13C NMR, ESI-HRMS, IR, element analysis, UV/Vis spectroscopy and fluorescence spectroscopy were performed to comprehensively characterize their chemical structures, spectral properties and stability. Nitrosamine impurities were not detected in 3a-3d, and the systemic toxicological assessment indicated that the toxicity of 3a-3d was lower. Furthermore, their anticancer activity was evaluated by MTT, AO/EB double staining, apoptosis detection and ROS detection. The time-dependent UV/Vis spectra revealed that 3a-3d had good stability in solution. For all the newly synthesized compounds, cytotoxic activities were carried out against human gastric cancer cell line BGC-823, human hepatoma cell line BEL-7402, human breast cancer cell line MCF-7 and human lung adenocarcinoma cell line A549 as well as human normal liver cell line HL-7702. MTT assay indicated that all the tested compounds exhibited important antiproliferative activity against selected cancer cell lines with IC50 values ranging from 7.01 to 34.32 μM, while none of them had obvious cytotoxic activity to human normal liver cell line HL-7702. Further, the most potent compound 3c displayed stronger antiproliferative activity against all the selected cancer cell lines than the clinically used anticancer agent 5-FU. Especially, 3b and 3c displayed cytotoxic activity against MCF-7 cells with IC50 values of 7.016 μM and 7.05 μM, respectively. AO/EB double staining, flow cytometry and ROS detection suggested that 3b and 3c could induce MCF-7 cell apoptosis in a dose-dependent manner. Molecular docking suggests that 3b and 3c could bind with DNA via partial insertion. Additionally, molecular docking also suggests that CDK2 may be one of the targets for 3b and 3c. In a word, 3b and 3c could be suitable candidates for further investigation as chemotherapeutic agents in cancer treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare that they have no relevant conflict of interest.
Figures


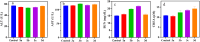




Comment in
-
Comment on "Design, synthesis, anticancer activity and molecular docking of quinoline-based dihydrazone derivatives" by J.-X. Lu, H.-R. Lan, D. Zeng, J.-Y. Song, Y.-T. Hao, A.-P. Xing, A. Shen, and J. Yuan, RSC Adv., 2025, 15, 231-243.RSC Adv. 2025 Nov 14;15(52):44437-44438. doi: 10.1039/d5ra00388a. eCollection 2025 Nov 11. RSC Adv. 2025. PMID: 41244744 Free PMC article.
References
-
- De Santana T. I. De Oliverira Barbosa M. De Moraes Gomes P. A. T. Da Cruz A. C. N. Da Silva T. G. Leite A. C. L. Eur. J. Med. Chem. 2018;144:874–886. - PubMed
-
- Wayteck L. Breckpot K. Demeester J. De Smedt S. C. Raemdonck K. Cancer Lett. 2014;352:113–125. - PubMed
-
- Lal K. Yadav P. Med. Chem. 2018;18:21–37. - PubMed
-
- Kwon S. Lee Y. Jung Y. Kim J. H. Baek B. Lim B. Lee J. Kim I. Lee J. Eur. J. Med. Chem. 2018;148:116–127. - PubMed
LinkOut - more resources
Full Text Sources

