Interleukin-2 receptor signaling acts as a checkpoint that influences the distribution of regulatory T cell subsets
- PMID: 39759017
- PMCID: PMC11700635
- DOI: 10.1016/j.isci.2024.111248
Interleukin-2 receptor signaling acts as a checkpoint that influences the distribution of regulatory T cell subsets
Abstract
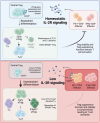
Regulatory T cells (Tregs) require IL-2 for survival in the periphery, yet how IL-2 shapes Treg heterogeneity remains poorly defined. Here we show that inhibition of IL-2R signaling in post-thymic Tregs leads to a preferential early loss of circulating Tregs (cTregs). Gene expression of cTregs was more dependent on IL-2R signaling than effector Tregs (eTregs). Unexpectedly, ablation of IL-2R signaling in cTregs resulted in increased proliferation, expression of eTreg genes, and enhanced capacity to develop into eTregs. Thus, IL-2R signaling normally acts as a checkpoint to maintain cTreg homeostasis while restraining their development into eTregs. Loss of IL-2R signaling also alters the distribution of eTreg subsets, with increased IFNγR1+ eTregs and CXCR5+ PD-1+ T follicular regulatory (TFR) cells but decreased intestinal RORγt+ TR17 cells. These changes lower eTreg suppressive function supporting expansion of IFNγ-secreting T effector cells. Thus, IL-2R signaling also safeguards Treg function and licenses differentiation of specialized eTregs.
Keywords: Biological sciences; Immune response; Immunology; Natural sciences.
© 2024 The Author(s).
Conflict of interest statement
The University of Miami and Thomas Malek have a patent on IL-2/CD25 fusion proteins and this technology has been licensed to Bristol Myers Squibb by the University of Miami for commercial development. Both receive royalties for its commercialization.
Figures








References
-
- Fontenot J.D., Rasmussen J.P., Gavin M.A., Rudensky A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

