Targeting CEA in metastatic triple negative breast cancer with image-guided radiation followed by Fab-mediated chimeric antigen receptor (CAR) T-cell therapy
- PMID: 39759518
- PMCID: PMC11695362
- DOI: 10.3389/fimmu.2024.1499471
Targeting CEA in metastatic triple negative breast cancer with image-guided radiation followed by Fab-mediated chimeric antigen receptor (CAR) T-cell therapy
Abstract
Introduction: Although CAR-T cell therapy has limited efficacy against solid tumors, it has been hypothesized that prior treatment with Image-Guided Radiation Therapy (IGRT) would increase CAR-T cell tumor infiltration, leading to improved antigen specific expansion of CAR-T cells.
Methods: To test this hypothesis in a metastatic triple negative breast cancer (TNBC) model, we engineered two anti-CEA single-chain Fab (scFab) CAR-T cells with signaling domains from CD28zeta and 4-1BBzeta, and tested them in vitro and in vivo.
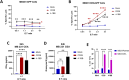
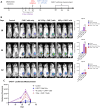
Results: The anti-CEA scFab CAR-T cells generated from three different human donors demonstrated robust in vitro expression, expansion, and lysis of only CEA-positive TNBC cells, with the CD28z-CAR-T cells showing the highest cytotoxicity. IFN-γ and granzyme B release assays revealed significantly higher IFN-γ production at a 4:1 effector-to-target (E:T) ratio in CD28z-CAR-T cells compared to 4-1BBz-CAR-T cells. Treatment of CEA-positive TNBC MDA-MB231 xenografts in the mammary fat pads of NSG mice, that produced spontaneous lung metastases over time, resulted in significant tumor growth reduction compared to either therapy alone (p<0.01). Immunohistochemical (IHC) analysis revealed that only combined IGRT and CAR-T therapy resulted in the elimination of lung metastases.
Discussion: These findings demonstrate that the combination of IGRT and anti-CEA scFab CAR-T therapy induces a strong antitumor response, effectively targeting both the primary tumor and distant metastatic lesions in the lungs, thus demonstrating that IGRT enhances CAR-T cell infiltration, persistence, and overall efficacy within both primary and metastatic lesions.
Keywords: carcinoembryonic antigen; cell immunotherapy; chimeric antigen receptor T cells; image-guided radiation therapy; triple-negative breast cancer.
Copyright © 2024 Aniogo, Kujawski, Awuah, Cha, Espinosa, Hui, Ghimire, Yazaki, Brown, Wang and Shively.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

