Evaluation of link between COVID-19 adjacent spike in hydroxychloroquine use and increased reports of pemphigus: a disproportionality analysis of the FDA Adverse Event Reporting System
- PMID: 39759530
- PMCID: PMC11695399
- DOI: 10.3389/fimmu.2024.1470660
Evaluation of link between COVID-19 adjacent spike in hydroxychloroquine use and increased reports of pemphigus: a disproportionality analysis of the FDA Adverse Event Reporting System
Abstract
Importance: Identifying environmental factors that contribute to disease onset/activity in PV stands to improve clinical outcomes and patient quality of life by strategies aimed at reducing specific disease promoting exposures and promoting personalized clinical management strategies.
Objective: To evaluate the association between hydroxychloroquine use and the development of pemphigus using population level, publicly available, FDA-generated data.
Design: Observational, retrospective, case-control, pharmacovigilance analysis.
Setting: Population based.
Participants: Individuals who either independently or via their healthcare provider submitted a voluntary report of a drug related adverse event to the FDA from Q4 of 2003 to Q2 of 2023.
Exposure: Cases were identified by the presence of adverse events described by the MedDRA preferred term (PT) of "pemphigus" (10034280) and then sorted based on exposure to the drug of interest, hydroxychloroquine, or lack thereof.
Main outcomes and measures: Frequency of hydroxychloroquine exposure among those individuals who reported an adverse event of pemphigus to the FDA; quantification of the reporting odds ratio (ROR).
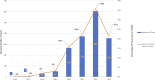
Results: We identified a total of 2,548 reports that included the adverse event pemphigus; among these, 1,545 (n=706 (41.92%) age 18-64, n=1 age 65-85 years, and n=977 (58.02%) with no age specified; n=1,366 (81.12%) females, n=4 (0.24%) males, and n=314 (18.65%) with no gender specified) included exposure to hydroxychloroquine (ROR, 282.647; 95% CI, 260.951-306.148). We then stratified those reports that included the combination of pemphigus and hydroxychloroquine by gender and found that while the association between the exposure and adverse event remained significant across genders, the magnitude of the effect sizes differed significantly (p<0.001), being over 100-fold greater among females (ROR, 378.7; 95% CI, 339.0-423.1) compared to males (ROR, 3.6; 95% CI, 1.4-9.8).
Conclusions and relevance: The frequency of reports containing the combination of the adverse event pemphigus and exposure to the drug hydroxychloroquine was disproportionately elevated across all genders in the years since the start of the COVID-19 pandemic. The disproportionately elevated frequency of reports of the combination of pemphigus and hydroxychloroquine supports an association between the two, corroborates previous case-report based evidence for such an association, suggests that hydroxychloroquine represents a possible trigger factor for the development of pemphigus, and paves the way for future research that is capable of establishing causality.
Keywords: COVID-19; FAERS; autoimmune bullous disease; autoimmunity; environmental factors; exposome; hydroxychloroquine; pemphigus.
Copyright © 2024 Baroukhian, Seiffert-Sinha, Attwood and Sinha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Center for Drug Evaluation and Research . FDA Adverse Event Reporting System (FAERS) Public Dashboard. FDA. Available online at: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-repor... (Accessed October 12, 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

