Reverse Phenotyping: Addressing Refractory Seizures From an Endocrine Perspective
- PMID: 39759686
- PMCID: PMC11699587
- DOI: 10.7759/cureus.75146
Reverse Phenotyping: Addressing Refractory Seizures From an Endocrine Perspective
Abstract
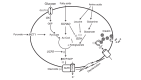
Neonatal hypoglycemia (NH) is a common abnormality in newborns, posing significant morbidity risks. Prompt diagnosis and treatment are vital to mitigate brain damage and enhance outcomes. Congenital hyperinsulinemia (CHI) is a leading cause of recurrent hypoglycemia in infants, often stemming from genetic mutations such as in the GLUD1 gene, manifesting as hyperinsulinism-hyperammonemia syndrome (HI/HA). We present a case of a 2-year-old girl with refractory epilepsy, later identified as HI/HA, whose paroxysmal episodes mimicked multiple seizure types. Genetic testing revealed a heterozygous pathogenic mutation in exon 2 of the GLUD1 gene. Treatment with diazoxide significantly improved blood sugar levels and achieved effective seizure control. Our case underscores the significance of considering metabolic etiologies like hyperinsulinemic hypoglycemia in children with seizures resistant to standard antiepileptic drugs. Early recognition, genetic testing, and targeted therapy are pivotal for achieving seizure control and optimizing patient outcomes.
Keywords: diazoxide; epilepsy; glud1; hyperinsulinism-hyperammonemia (hi/ha) syndrome; neurodevelopmental disorders.
Copyright © 2024, Sherin et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
