Metal-phenolic network biointerface-mediated cell regulation for bone tissue regeneration
- PMID: 39759849
- PMCID: PMC11699301
- DOI: 10.1016/j.mtbio.2024.101400
Metal-phenolic network biointerface-mediated cell regulation for bone tissue regeneration
Abstract
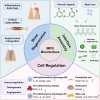
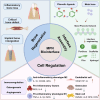
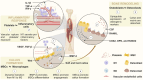
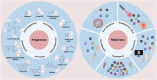
Bone tissue regeneration presents a significant challenge in clinical treatment due to inadequate coordination between implant materials and reparative cells at the biomaterial-bone interfaces. This gap underscores the necessity of enhancing interaction modulation between cells and biomaterials, which is a crucial focus in bone tissue engineering. Metal-polyphenolic networks (MPN) are novel inorganic-organic hybrid complexes that are formed through coordination interactions between phenolic ligands and metal ions. These networks provide a multifunctional platform for biomedical applications, with the potential for tailored design and modifications. Despite advances in understanding MPN and their role in bone tissue regeneration, a comprehensive overview of the related mechanisms is lacking. Here, we address this gap by focusing on MPN biointerface-mediated cellular regulatory mechanisms during bone regeneration. We begin by reviewing the natural healing processes of bone defects, followed by a detailed examination of MPN, including their constituents and distinctive characteristics. We then explore the regulatory influence of MPN biointerfaces on key cellular activities during bone regeneration. Additionally, we illustrate their primary applications in addressing inflammatory bone loss, regenerating critical-size bone defects, and enhancing implant-bone integration. In conclusion, this review elucidates how MPN-based interfaces facilitate effective bone tissue regeneration, advancing our understanding of material interface-mediated cellular control and the broader field of tissue engineering.
Keywords: Biointerface; Bone tissue regeneration; Immunoregulation; Metal-polyphenolic networks; Stem cells.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Feng P., Zhao R., Tang W., Yang F., Tian H., Peng S., et al. Structural and functional adaptive artificial bone: materials, fabrications, and properties. Adv. Funct. Mater. 2023;33(23)
-
- Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. - PubMed
-
- Hao J., Malek N.A.N.N., Kamaruddin W.H.A., Li J. Breaking piezoelectric limits of molecules for biodegradable implants. BME Mat. 2024;2(2)
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

