Molecular Characterization of Gene Encoding Outer Membrane Protein loa22 in Pathogenic Leptospira Serovars in Iran
- PMID: 39760050
- PMCID: PMC11699983
- DOI: 10.1155/jotm/3900663
Molecular Characterization of Gene Encoding Outer Membrane Protein loa22 in Pathogenic Leptospira Serovars in Iran
Abstract
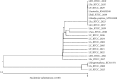
The loa22 protein is highly conserved among pathogenic Leptospira serovars and it is expressed during both acute and chronic infections. The aim of this study was to clone and sequence of the loa22 protein-encoding gene of Leptospira serovars. In this study, 23 pathogenic Leptospira serovars and two nonpathogenic Leptospira serovars were used. These serovars were obtained from the microbial culture collection of Leptospira Reference Laboratory, Department of Microbiology, Razi Vaccine and Serum Research Institute, Karaj, Iran. Three serovars, including L. Sejroe Hardjo-bovis, L. Grippotyphosa, L. Canicola, are used in the preparation of the trivalent vaccine. The loa22 gene was amplified by specific primers and the PCR products were then purified using kit and were cloned into a pTZ57R/T vector and transformed in competent E. coli DH5α cells. The cells were then plated onto LB agar containing ampicillin and recombinant colonies subjected to colony PCR to confirm the presence of the Leptospiral gene. Positive colonies plasmid vector was isolated from cells by High Pure Plasmid Isolation Kit. The loa22 gene was detected in all 23 pathogenic serovars, while this gene was not observed in nonpathogenic L. biflexa. It was determined that the similarity percentage of the sequenced pathogenic serovars is between 95.5% and 100%. The results concluded that the loa22 gene was highly conserved among various pathogenic Leptospira serovars and can be used to develop an effective recombinant vaccine.
Keywords: cloning; leptospirosis; loa22 gene; pathogenic serovars; sequencing.
Copyright © 2024 Yeganeh Malek Mohammadi et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Kurilung A., Keeratipusana C., Suriyaphol P., Hampson D. J., Prapasarakul N. Genomic Analysis of Leptospira Interrogans Serovar Paidjan and Dadas Isolates From Carrier Dogs and Comparative Genomic Analysis to Detect Genes Under Positive Selection. BMC Genomics . 2019;20(1):168–219. doi: 10.1186/s12864-019-5562-z. - DOI - PMC - PubMed
-
- Khaki P. Clinical Laboratory Diagnosis of Human Leptospirosis. International Journal of Enteric Pathogens . 2016;4(1):1–7. doi: 10.17795/ijep31859. - DOI
-
- Zavitsanou A., Babatsikou F. Leptospirosis: Epidemiology and Preventive Measures. Health Science Journal . 2008;2(2):75–82.
LinkOut - more resources
Full Text Sources

