A deformable SIS/HA composite hydrogel coaxial scaffold promotes alveolar bone regeneration after tooth extraction
- PMID: 39760069
- PMCID: PMC11697370
- DOI: 10.1016/j.bioactmat.2024.12.008
A deformable SIS/HA composite hydrogel coaxial scaffold promotes alveolar bone regeneration after tooth extraction
Abstract
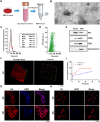
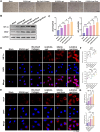
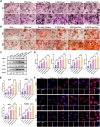
After tooth extraction, alveolar bone absorbs unevenly, leading to soft tissue collapse, which hinders full regeneration. Bone loss makes it harder to do dental implants and repairs. Inspired by the biological architecture of bone, a deformable SIS/HA (Small intestinal submucosa/Hydroxyapatite) composite hydrogel coaxial scaffold was designed to maintain bone volume in the socket. The SIS/HA scaffold containing GL13K as the outer layer, mimicking compact bone, while SIS hydrogel loaded with bone marrow mesenchymal stem cells-derived exosomes (BMSCs-Exos) was utilized as the inner core of the scaffolds, which are like soft tissue in the skeleton. This coaxial scaffold exhibited a modulus of elasticity of 0.82 MPa, enabling it to adaptively fill extraction sockets and maintain an osteogenic space. Concurrently, the inner layer of this composite scaffold, enriched with BMSCs-Exos, promoted the proliferation and migration of human umbilical vein endothelial cells (HUVECs) and BMSCs into the scaffold interior (≈3-fold to the control), up-regulated the expression of genes related to osteogenesis (BMP2, ALP, RUNX2, and OPN) and angiogenesis (HIF-1α and VEGF). This induced new blood vessels and bone growth within the scaffold, addressing the issue of low bone formation rates at the center of defects. GL13K was released by approximately 40.87 ± 4.37 % within the first three days, exerting a localized antibacterial effect and further promoting vascularization and new bone formation in peripheral regions. This design aims to achieve an all-around and efficient bone restoration effect in the extraction socket using coaxial scaffolds through a dual internal and external mechanism.
Keywords: Alveolar ridge preservation; Angiogenesis; Antibacterial; Exosome; Osteogenesis; SIS/HA.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following personal relationships which may be considered as potential competing interests: Pengfei Wei and Bo Zhao are currently employed by Beijing Biosis Healing Biological Technology Co., Ltd.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials

