Nonviral targeted mRNA delivery: principles, progresses, and challenges
- PMID: 39760110
- PMCID: PMC11695212
- DOI: 10.1002/mco2.70035
Nonviral targeted mRNA delivery: principles, progresses, and challenges
Abstract
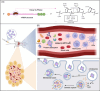
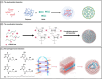
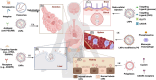
Messenger RNA (mRNA) therapeutics have garnered considerable attention due to their remarkable efficacy in the treatment of various diseases. The COVID-19 mRNA vaccine and RSV mRNA vaccine have been approved on the market. Due to the inherent nuclease-instability and negative charge of mRNA, delivery systems are developed to protect the mRNA from degradation and facilitate its crossing cell membrane to express functional proteins or peptides in the cytoplasm. However, the deficiency in transfection efficiency and targeted biological distribution are still the major challenges for the mRNA delivery systems. In this review, we first described the physiological barriers in the process of mRNA delivery and then discussed the design approach and recent advances in mRNA delivery systems with an emphasis on their tissue/cell-targeted abilities. Finally, we pointed out the existing challenges and future directions with deep insights into the design of efficient mRNA delivery systems. We believe that a high-precision targeted delivery system can greatly improve the therapeutic effects and bio-safety of mRNA therapeutics and accelerate their clinical transformations. This review may provide a new direction for the design of mRNA delivery systems and serve as a useful guide for researchers who are looking for a suitable mRNA delivery system.
Keywords: delivery obstacles; mRNA therapeutics; nonviral delivery; targeted delivery systems.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Optimal delivery strategies for nanoparticle-mediated mRNA delivery.J Mater Chem B. 2023 Mar 8;11(10):2063-2077. doi: 10.1039/d2tb02455a. J Mater Chem B. 2023. PMID: 36794598 Review.
-
Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems.Gene Ther. 2017 Mar;24(3):133-143. doi: 10.1038/gt.2017.5. Epub 2017 Jan 17. Gene Ther. 2017. PMID: 28094775 Review.
-
Inhaled non-viral delivery systems for RNA therapeutics.Acta Pharm Sin B. 2025 May;15(5):2402-2430. doi: 10.1016/j.apsb.2025.03.033. Epub 2025 Mar 19. Acta Pharm Sin B. 2025. PMID: 40487670 Free PMC article. Review.
-
Complex Coacervates as a Promising Vehicle for mRNA Delivery: A Comprehensive Review of Recent Advances and Challenges.Mol Pharm. 2023 Sep 4;20(9):4387-4403. doi: 10.1021/acs.molpharmaceut.3c00439. Epub 2023 Aug 10. Mol Pharm. 2023. PMID: 37561647 Review.
-
Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing.Acc Chem Res. 2021 Nov 2;54(21):4001-4011. doi: 10.1021/acs.accounts.1c00500. Epub 2021 Oct 20. Acc Chem Res. 2021. PMID: 34668716 Review.
Cited by
-
Recent Advances in mRNA-Based Vaccines Against Several Hepatitis Viruses.Biol Proced Online. 2025 Jun 3;27(1):20. doi: 10.1186/s12575-025-00269-2. Biol Proced Online. 2025. PMID: 40461976 Free PMC article. Review.
References
-
- Cobb M. Who discovered messenger RNA? Curr Biol. 2015;25(13):R526‐32. - PubMed
-
- Sahin U, Karikó K, Türeci Ö. mRNA‐based therapeutics — developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759‐780. - PubMed
-
- Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949):1465‐1468. - PubMed
-
- Hecker JG. Non‐viral, lipid‐mediated DNA and mRNA gene therapy of the central nervous system (CNS): chemical‐based transfection. Methods Mol Biol. 2016;1382:307‐324. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
