Elevated developmental temperatures below the lethal limit reduce Aedes aegypti fertility
- PMID: 39760305
- PMCID: PMC11832123
- DOI: 10.1242/jeb.249803
Elevated developmental temperatures below the lethal limit reduce Aedes aegypti fertility
Abstract
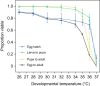
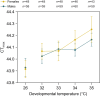
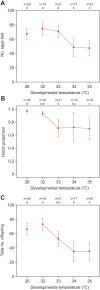
Aedes aegypti mosquitoes are the principal vectors of dengue and continue to pose a threat to human health, with ongoing urbanization, climate change and trade all impacting the distribution and abundance of this species. Hot periods are becoming increasingly common and their impacts on insect mortality have been well established, but they may have even greater impacts on insect fertility. In this study, we investigated the impacts of high temperatures on Ae. aegypti fertility both within and across generations. Mosquitoes developing under elevated temperatures exhibited higher critical thermal maxima (CTmax), reflecting developmental acclimation, but their fertility declined with increasing developmental temperature. In females, elevated developmental temperatures decreased fecundity while in males it tended to decrease the proportion of eggs that hatched and the proportion of individuals producing viable offspring. Rearing both sexes at 35°C increased fecundity in the subsequent generation but effects of elevated temperatures persisted across gonotrophic cycles within the same generation. Moreover, exposure of adults to 35°C further decreased fertility beyond the effects of developmental temperature alone. These findings highlight sub-lethal impacts of elevated temperatures on Ae. aegypti fertility and plastic responses to thermal stress within and across generations. This has significant implications for predicting the distribution and abundance of mosquito populations thriving in increasingly warmer environments.
Keywords: Acclimation; Fecundity; Heat stress; Mosquito; Thermal limit.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Thermal influence on development and morphological traits of Aedes aegypti in central India and its relevance to climate change.Parasit Vectors. 2025 Jul 11;18(1):279. doi: 10.1186/s13071-025-06924-7. Parasit Vectors. 2025. PMID: 40646649 Free PMC article.
-
Impact of temperature on survival, development and longevity of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Phnom Penh, Cambodia.Parasit Vectors. 2025 Aug 27;18(1):362. doi: 10.1186/s13071-025-06892-y. Parasit Vectors. 2025. PMID: 40867007 Free PMC article.
-
Effects of desiccation stress on adult female longevity in Aedes aegypti and Ae. albopictus (Diptera: Culicidae): results of a systematic review and pooled survival analysis.Parasit Vectors. 2018 Apr 25;11(1):267. doi: 10.1186/s13071-018-2808-6. Parasit Vectors. 2018. PMID: 29695282 Free PMC article.
-
The thermal stress response of Aedes aegypti and Aedes albopictus when exposed to rapid temperature changes.Parasit Vectors. 2025 Jul 26;18(1):300. doi: 10.1186/s13071-025-06951-4. Parasit Vectors. 2025. PMID: 40713850 Free PMC article.
-
Wolbachia-carrying Aedes mosquitoes for preventing dengue infection.Cochrane Database Syst Rev. 2024 Apr 10;4(4):CD015636. doi: 10.1002/14651858.CD015636.pub2. Cochrane Database Syst Rev. 2024. PMID: 38597256 Free PMC article.
References
-
- Agyekum, T. P., Botwe, P. K., Arko-Mensah, J., Issah, I., Acquah, A. A., Hogarh, J. N., Dwomoh, D., Robins, T. G. and Fobil, J. N. (2021). A systematic review of the effects of temperature on Anopheles mosquito development and survival: implications for malaria control in a future warmer climate. Int. J. Environ. Res. Public Health 18, 7255. 10.3390/ijerph18147255 - DOI - PMC - PubMed
-
- Bader, C. A. and Williams, C. R. (2012). Mating, ovariole number and sperm production of the dengue vector mosquito Aedes aegypti(L.) in Australia: broad thermal optima provide the capacity for survival in a changing climate. Physiol. Entomol. 37, 136-144. 10.1111/j.1365-3032.2011.00818.x - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

