Depletion of TP53 in Human Pluripotent Stem Cells Triggers Malignant-Like Behavior
- PMID: 39760438
- PMCID: PMC12001006
- DOI: 10.1002/adbi.202400538
Depletion of TP53 in Human Pluripotent Stem Cells Triggers Malignant-Like Behavior
Abstract
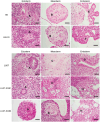
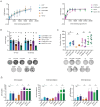
Human pluripotent stem cells (hPSCs) tend to acquire genetic aberrations upon culture in vitro. Common aberrations are mutations in the tumor suppressor TP53, suspected to confer a growth-advantage to the mutant cells. However, their full impact in the development of malignant features and safety of hPSCs for downstream applications is yet to be elucidated. Here, TP53 is knocked out in hPSCs using CRISPR-Cas9 and compared them with isogenic wild-type hPSCs and human germ cell tumor lines as models of malignancy. While no major changes in proliferation, pluripotency, and transcriptomic profiles are found, mutant lines display aberrations in some of the main chromosomal hotspots for genetic abnormalities in hPSCs. Additionally, enhanced clonogenic and anchorage-free growth, alongside resistance to chemotherapeutic compounds is observed. The results indicate that common TP53-depleting mutations in hPSCs, although potentially overlooked by standard analyses, can impact their behavior and safety in a clinical setting.
Keywords: TP53; genetic aberration; human germ cell tumor; human pluripotent stem cell; malignancy; pluripotency.
© 2025 The Author(s). Advanced Biology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells.Nat Med. 2018 Jul;24(7):939-946. doi: 10.1038/s41591-018-0050-6. Epub 2018 Jun 11. Nat Med. 2018. PMID: 29892062
-
P53 Regulates Rapid Apoptosis in Human Pluripotent Stem Cells.J Mol Biol. 2016 Apr 10;428(7):1465-75. doi: 10.1016/j.jmb.2015.07.019. Epub 2015 Jul 31. J Mol Biol. 2016. PMID: 26239243 Free PMC article.
-
Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations.Nature. 2017 May 11;545(7653):229-233. doi: 10.1038/nature22312. Epub 2017 Apr 26. Nature. 2017. PMID: 28445466 Free PMC article.
-
CRISPR/Cas9 genome editing in human pluripotent stem cells: Harnessing human genetics in a dish.Dev Dyn. 2016 Jul;245(7):788-806. doi: 10.1002/dvdy.24414. Epub 2016 Jun 9. Dev Dyn. 2016. PMID: 27145095 Review.
-
Pipeline for the Generation and Characterization of Transgenic Human Pluripotent Stem Cells Using the CRISPR/Cas9 Technology.Cells. 2020 May 25;9(5):1312. doi: 10.3390/cells9051312. Cells. 2020. PMID: 32466123 Free PMC article. Review.
Cited by
-
Yolk Sac Elements in Tumors Derived from Pluripotent Stem Cells: Borrowing Knowledge from Human Germ Cell Tumors.Int J Mol Sci. 2025 Jul 4;26(13):6464. doi: 10.3390/ijms26136464. Int J Mol Sci. 2025. PMID: 40650246 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

