Superoxide dismutase 2 deficiency in mesenchymal stromal cells induces sympathetic denervation and functional impairment of brown adipose tissue
- PMID: 39760485
- PMCID: PMC11848962
- DOI: 10.1111/pin.13503
Superoxide dismutase 2 deficiency in mesenchymal stromal cells induces sympathetic denervation and functional impairment of brown adipose tissue
Abstract
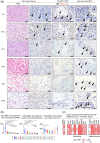
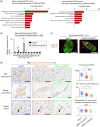
Brown adipose tissue (BAT) is an energy-consuming organ, and its functional dysregulation contributes to the development of metabolic diseases and obesity. BAT function is regulated by the sympathetic nervous system but declines with age, which is partly caused by reduced sympathetic nerve fibers innervating BAT. Thus far, the role of mesenchymal stromal/stem cells in age-related BAT dysfunction remains unknown. Here, we show that BAT dysfunction may be induced by a defect in the antioxidant capacity of stromal cells that localize in and around the nerve fibers (perineurial cells) of BAT. These cells express Meflin, a marker of mesenchymal stromal/stem cells. Specific deletion of the antioxidant enzyme superoxide dismutase 2 in Meflin-lineage cells caused sympathetic denervation and whitening of BAT and its functional impairment, as exemplified by a decline in the fat oxidation rate during the daytime. This phenotype was accompanied by overexpression of the neurorepulsive factor semaphorin 3A in perineurial cells. Notably, Meflin-deficient mice exhibited resistance to doxorubicin-induced BAT dysfunction. These results highlight the role of Meflin+ stromal cells, including perineurial cells, in maintaining BAT function and suggest that targeting BAT stromal cells provides a new avenue for improving BAT function.
Keywords: ISLR; Meflin; brown adipose tissue; oxidative stress; perineurial cells; semaphorin 3A.
© 2025 The Author(s). Pathology International published by Japanese Society of Pathology and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Atsushi Enomoto and Shinji Mii are Editorial Board members of Pathology International and co‐authors of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication. All other authors declare no conflict of interest.
Figures




References
-
- Graja A, Schulz TJ. Mechanisms of aging‐related impairment of brown adipocyte development and function. Gerontology. 2015;61:211–217. - PubMed
-
- Scarpace PJ, Mooradian AD, Morley JE. Age‐associated decrease in beta‐adrenergic receptors and adenylate cyclase activity in rat brown adipose tissue. J Gerontol. 1988;43:B65–B70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

