Perinatal outcomes among pregnant women with HIV initiating antiretroviral therapy preconception and antenatally
- PMID: 39760703
- PMCID: PMC11902611
- DOI: 10.1097/QAD.0000000000004104
Perinatal outcomes among pregnant women with HIV initiating antiretroviral therapy preconception and antenatally
Abstract
Objective: Increasingly, pregnant women with HIV (WHIV) initiate antiretroviral therapy (ART) before conception. We assessed the risk of adverse perinatal outcomes among pregnant WHIV initiating ART preconception or antenatally, compared with women without HIV or ART-naive WHIV.
Design: Systematic review and meta-analysis.
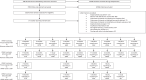
Methods: We searched PubMed, EMBASE, CINAHL, and Global Health for studies published between 1 January 1980 and 14 July 2023. We assessed the association of preconception/antenatal ART initiation with preterm birth (PTB), very PTB (VPTB), spontaneous PTB (sPTB), low birthweight (LBW), very LBW (VLBW), small for gestational age (SGA), very SGA (VSGA), stillbirth and neonatal death (NND). Data were analysed using random effects meta-analyses. Quality assessments, subgroup and sensitivity analyses were conducted. PROSPERO registration: CRD42021248987.
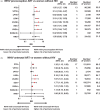
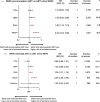
Results: Thirty-one cohort studies were eligible, including 199 156 women in 19 countries. WHIV with preconception ART were associated with increased risk of PTB [risk ratio (RR) 1.55; 95% confidence interval (CI) 1.27-1.90], VPTB (RR 2.14, 95% CI 1.02-4.47), LBW (RR 2.19, 95% CI 1.32-3.63), VLBW (RR 3.34, 95% CI 1.08-10.35), SGA (RR 1.92, 95% CI 1.01-3.66), and VSGA (RR 2.79, 95% CI 1.04-7.47), compared with women without HIV. WHIV with antenatal ART were associated with increased risk of PTB (RR 1.35, 95% CI 1.15-1.58), LBW (RR 2.16, 95% CI 1.39-3.34), VLBW (RR 1.97, 95% CI 1.01-3.84), SGA (RR 1.77, 95% CI 1.10-2.84), and VSGA (RR 1.21, 95% CI 1.09-1.33), compared with women without HIV. Compared to ART-naive WHIV, WHIV with preconception or antenatal ART were associated with increased risk of SGA (preconception: RR 1.40, 95% CI 1.12-1.73; antenatal: RR 1.39, 95% CI 1.11-1.74) and VSGA (preconception: RR 2.44, 95% CI 1.63-3.66; antenatal: RR 2.24, 95% CI 1.48-3.40).
Conclusion: Among WHIV, both preconception and antenatal initiation of ART are associated with increased risks of adverse perinatal outcomes, compared to women without HIV and ART-naive WHIV.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Adverse perinatal outcomes associated with different classes of antiretroviral drugs in pregnant women with HIV.AIDS. 2025 Feb 1;39(2):162-174. doi: 10.1097/QAD.0000000000004032. Epub 2024 Oct 15. AIDS. 2025. PMID: 39407417 Free PMC article.
-
Adverse perinatal outcomes associated with protease inhibitor-based antiretroviral therapy in pregnant women living with HIV: A systematic review and meta-analysis.EClinicalMedicine. 2022 Apr 6;46:101368. doi: 10.1016/j.eclinm.2022.101368. eCollection 2022 Apr. EClinicalMedicine. 2022. PMID: 35521067 Free PMC article.
-
Adverse perinatal outcomes associated with prenatal exposure to protease-inhibitor-based versus non-nucleoside reverse transcriptase inhibitor-based antiretroviral combinations in pregnant women with HIV infection: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2023 Jan 30;23(1):80. doi: 10.1186/s12884-023-05347-5. BMC Pregnancy Childbirth. 2023. PMID: 36717801 Free PMC article.
-
Adverse perinatal outcomes associated with timing of initiation of antiretroviral therapy: Systematic review and meta-analysis.HIV Med. 2023 Feb;24(2):111-129. doi: 10.1111/hiv.13326. Epub 2022 Jun 6. HIV Med. 2023. PMID: 35665582
-
Adverse perinatal outcomes associated with HAART and monotherapy.AIDS. 2022 Aug 1;36(10):1409-1427. doi: 10.1097/QAD.0000000000003248. Epub 2022 May 25. AIDS. 2022. PMID: 35608111
References
-
- UNAIDS. The urgency of now: AIDS at a crossroads. Geneva Joint United Nations Programme on HIV/AIDS, 2024.
-
- Doherty M, Ford N, Vitoria M, Weiler G, Hirnschall G. The 2013 WHO guidelines for antiretroviral therapy: evidence-based recommendations to face new epidemic realities. Curr Opin HIV AIDS 2013; 8:528–534. - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva: WHO; 2016. - PubMed
-
- World Health Organization. Estimated percentage of pregnant women living with HIV who received antiretrovirals for preventing mother-to-child transmission. Geneva: WHO; 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

