ALG5 downregulation inhibits osteogenesis and promotes adipogenesis by regulating the N-glycosylation of SLC6A9 in osteoporosis
- PMID: 39760914
- PMCID: PMC11703790
- DOI: 10.1007/s00018-024-05566-9
ALG5 downregulation inhibits osteogenesis and promotes adipogenesis by regulating the N-glycosylation of SLC6A9 in osteoporosis
Abstract
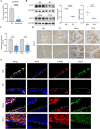
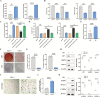
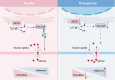
Osteoporosis is characterized by decreased bone mass and accumulation of adipocytes in the bone marrow. The mechanism underlying the imbalance between osteoblastogenesis and adipogenesis in bone marrow mesenchymal stem cells (BMSCs) remains unclear. We found that ALG5 was significantly downregulated in BMSCs from osteoporotic specimens. ALG5 knockdown inhibited osteogenic differentiation and increased adipogenic differentiation of BMSCs. ALG5 deficiency diminished the N-glycosylation of SLC6A9, thereby altering its protein stability and disrupting SLC6A9-mediated glycine uptake in BMSCs. ALG5 overexpression by adeno-associated virus serotype 9 (rAAV9) alleviated bone loss in OVX mice. Taken together, our findings suggest a novel role for the ALG5-SLC6A9-glycine axis in the imbalance of BMSC differentiation in osteoporosis. Moreover, we identify ALG5 overexpression as a potential therapeutic strategy for treating osteoporosis.
Keywords: ALG5; Bone marrow mesenchymal stem cells; Glycine; Osteoporosis; SLC6A9.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors declare that they have no competing interests. Ethical approval: The animal experimentation protocols received approval from the Institutional Animal Care and Use Committee of Sun Yat-Sen University. The assigned approval numbers for the experiments were 2022003644, 2023000742, and 2023001223. Additionally, collection and experimentation involving human specimens were ethically approved by the Ethics Committee of the Eighth Affiliated Hospital, Sun Yat-Sen University, with the respective approval numbers for human subject handling being 2021r037 and 2022r023. Consent for publication: All authors have read and approved the manuscript.
Figures




Similar articles
-
LncRNA SNHG14 Delivered by Bone Marrow Mesenchymal Stem Cells-Secreted Exosomes Regulates Osteogenesis and Adipogenesis in Osteoporosis by Mediating the miR-27a-3p/LMNB1 Axis.Kaohsiung J Med Sci. 2025 May;41(5):e70004. doi: 10.1002/kjm2.70004. Epub 2025 Mar 7. Kaohsiung J Med Sci. 2025. PMID: 40052307 Free PMC article.
-
Circular RNA circSTX12 regulates osteo-adipogenic balance and proliferation of BMSCs in senile osteoporosis.Cell Mol Life Sci. 2025 Apr 7;82(1):149. doi: 10.1007/s00018-025-05684-y. Cell Mol Life Sci. 2025. PMID: 40192802 Free PMC article.
-
MiR-210 promotes bone formation in ovariectomized rats by regulating osteogenic/adipogenic differentiation of bone marrow mesenchymal stem cells through downregulation of EPHA2.J Orthop Surg Res. 2023 Oct 30;18(1):811. doi: 10.1186/s13018-023-04213-6. J Orthop Surg Res. 2023. PMID: 37904187 Free PMC article.
-
FoxO3 Regulates Mouse Bone Mesenchymal Stem Cell Fate and Bone-Fat Balance During Skeletal Aging.Stem Cells Dev. 2024 Jul;33(13-14):365-375. doi: 10.1089/scd.2024.0055. Epub 2024 May 13. Stem Cells Dev. 2024. PMID: 38661524
-
The miR-665/SOST Axis Regulates the Phenotypes of Bone Marrow Mesenchymal Stem Cells and Osteoporotic Symptoms in Female Mice.Am J Pathol. 2024 Nov;194(11):2059-2075. doi: 10.1016/j.ajpath.2024.07.022. Am J Pathol. 2024. PMID: 39461772
References
MeSH terms
Grants and funding
- 2023B110001/Guangdong Provincial Clinical Research Center for Orthopedic Diseases
- YXYXCXRC202101/Excellent Medical Innovation Talent Program of the Eighth Affiliated Hospital of Sun Yat-sen University
- 82172349/National Natural Science Foundation of China
- 82372372/National Natural Science Foundation of China
- 82302661/National Natural Science Foundation of China
- 2023A1515010568/Guangdong Natural Science Foundation
- 2021A1515111057/Guangdong Natural Science Foundation
- JCYJ20220530144201004/Shenzhen Science and Technology Program
- RCBS20210609104445097/Shenzhen Science and Technology Program
- FTWS2022022/Futian Healthcare Research Project
- FTWS2021013/Futian Healthcare Research Project
- FTWS2023072/Futian Healthcare Research Project
- FTWS2022047/Futian Healthcare Research Project
LinkOut - more resources
Full Text Sources
Medical

