Evaluation of Three Viral Capsid Integrity qPCR Methods for Wastewater-Based Viral Surveillance
- PMID: 39760935
- PMCID: PMC11703991
- DOI: 10.1007/s12560-024-09627-x
Evaluation of Three Viral Capsid Integrity qPCR Methods for Wastewater-Based Viral Surveillance
Abstract
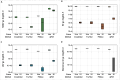
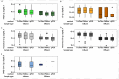
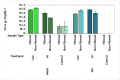
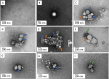
Capsid Integrity qPCR (CI-qPCR) assays offer a promising alternative to cell culture-based infectivity assays for assessing pathogenic human virus viability in wastewater. This study compared three CI-qPCR methods: two novel (Crosslinker, TruTiter) and one established (PMAxx dye). These methods were evaluated on heat-inactivated and non-heat-inactivated 'live' viruses spiked into phosphate-buffered saline (PBS) and wastewater, as well as on viruses naturally present in wastewater samples. The viral panel included Human adenovirus 5 (HAdV), enterovirus A71 (EV), hepatitis-A virus (HAV), influenza-A H3N2 (IAV), respiratory syncytial virus A2 (RSV), norovirus GI, norovirus GII, and SARS-CoV-2. All three methods successfully differentiated between degraded, heat-inactivated, and live viruses in PBS. While all three methods were comparable for HAdV and norovirus GI, PMAxx detected significantly lower gene copies for EV and IAV. In spiked wastewater, PMAxx yielded significantly lower gene copies for all heat-inactivated viruses (HAdV, EV, HAV, IAV, and RSV) compared to the Crosslinker and TruTiter methods. For viruses naturally present in wastewater (un-spiked), no significant difference was observed between PMAxx and TruTiter methods. Intact, potentially infectious viruses were detected using both PMAxx and TruTiter on untreated and treated wastewater samples. A comparative analysis of qPCR data and TEM images revealed that viral flocculation of IAV may interfere with capsid integrity assays using intercalating dyes. In summary, our findings not only advance the development of more effective methods for assessing viral viability in wastewater, but also highlight the potential of CI-qPCR techniques to enhance early warning systems for emerging pathogens, thereby strengthening public health preparedness and response strategies.
Keywords: Capsid integrity; Environmental surveillance; Pathogen viability; Public health risk; Viral persistence; Wastewater-based epidemiology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures

References
-
- Agrawal, S., Orschler, L., Schubert, S., Zachmann, K., Heijnen, L., Tavazzi, S., Gawlik, B. M., de Graaf, M., Medema, G., & Lackner, S. (2022). Prevalence and circulation patterns of SARS-CoV-2 variants in European sewage mirror clinical data of 54 European cities. Water Research,214, 118162. 10.1016/J.WATRES.2022.118162 - DOI - PMC - PubMed
-
- Ahmed, W., Angel, N., Edson, J., Bibby, K., Bivins, A., Obrien, J. W., Choi, P. M., Kitajima, M., Simpson, S. L., Li, J., Tscharke, B., Verhagen, R., Smith, W. J. M., Zaugg, J., Dierens, L., Hugenholtz, P., Thomas, K. V., & Mueller, J. F. (2020). First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Science of the Total Environment. 10.1016/j.scitotenv.2020.138764 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

