A Maximum-Use Trial of Ruxolitinib Cream in Children Aged 2-11 Years with Moderate to Severe Atopic Dermatitis
- PMID: 39760983
- PMCID: PMC11850462
- DOI: 10.1007/s40257-024-00909-5
A Maximum-Use Trial of Ruxolitinib Cream in Children Aged 2-11 Years with Moderate to Severe Atopic Dermatitis
Abstract
Background: Ruxolitinib cream has demonstrated anti-inflammatory and antipruritic activity and was well tolerated in a phase 3 study in patients aged 2-11 years with mild to moderate atopic dermatitis (AD).
Objective: This study examined the safety, tolerability, pharmacokinetics, efficacy, and quality of life (QoL) with ruxolitinib cream under maximum-use conditions and with longer-term use.
Methods: Eligible patients were aged 2-11 years with moderate to severe AD [Investigator's Global Assessment (IGA) score 3-4], and ≥ 35% affected body surface area (BSA). Patients applied 1.5% ruxolitinib cream twice daily to all baseline-identified lesions during the 4-week maximum-use period, then to active lesions only up to week 52 (patients with ≤ 20% affected BSA from week 8). Safety was assessed by frequency and severity of adverse events. Pharmacokinetic parameters were assessed as secondary endpoints, and efficacy and QoL were exploratory endpoints.
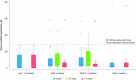
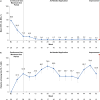
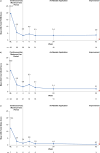
Results: Overall, 29 patients (median age 5 years) were enrolled. Treatment-emergent adverse events were reported in 9/29 patients (31.0%); there were no adverse events of special interest (i.e., no serious infections, malignancies, major adverse cardiovascular events, or thromboses) during the study period. Mean steady-state plasma concentration during the maximum-use period was below the known half-maximal inhibitory concentration of Janus kinase-mediated myelosuppression in adults. Reductions in affected BSA and IGA observed at week 4 were sustained with as-needed use through 52 weeks. Improvements in patient-reported outcomes and QoL measures were consistent with efficacy results.
Conclusion: These results support the safety of ruxolitinib cream in children (2-11 years) with AD, including those with extensive disease, and are consistent with previous efficacy findings.
Gov identifier: NCT05034822, first registered 30 August 2021.
Plain language summary
Ruxolitinib cream 1.5% is approved in the USA for patients aged ≥ 12 years for the treatment of mild to moderate atopic dermatitis (AD) involving ≤ 20% of the body, with recent studies supporting the safety and efficacy of ruxolitinib cream in younger children with mild to moderate AD. Maximum-use trials look at the safety of treatments applied to more extensive areas of skin, assessing potential for side effects. This maximum-use trial assessed safety, absorption, and effectiveness of 1.5% ruxolitinib cream when applied twice daily for 4 weeks in children aged 2–11 years with AD involving ≥ 35% of the body. Patients then applied ruxolitinib cream as needed for ≤ 52 weeks, and safety and disease control were assessed. During the first 4 weeks, 31% of patients reported side effects. Only one patient experienced treatment-related side effects at the application site. As-needed ruxolitinib cream did not cause any other side effects of concern. The average ruxolitinib blood level during the first 4 weeks was low. As expected with low ruxolitinib blood levels, no side effects associated with oral drugs of the same class (e.g., low levels of white blood cells, serious infections, cancers, major cardiovascular events, or blood clots) were seen. AD lesions decreased in size and there was relief of itching during the first 4 weeks of treatment, and the amount of ruxolitinib cream applied decreased thereafter. Disease control was maintained for ≤ 52 weeks with as-needed ruxolitinib cream. These findings help to confirm the safety and effectiveness of ruxolitinib cream in children.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study was funded by Incyte Corporation (Wilmington, DE, USA). Competing Interests: LSG has served as an investigator, advisor, and/or speaker for AbbVie, Arcutis, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi. RB is an advisory board member, consultant, speaker, and/or investigator for and received honoraria and/or grants from AbbVie, Almirall, Amgen, AnaptysBio, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Escalier, Janssen, Kyowa Kirin, LEO Pharma, Nimbus, Pfizer, Regeneron, Sienna, and UCB. He is also an employee and shareholder of Innovaderm Research. SF has received honoraria, clinical research grants, or fees as a consultant, speaker, advisory board member, and/or investigator for AbbVie, Aclaris Therapeutics, Asana BioSciences, AstraZeneca, Athenex, Celgene Corporation, Cutanea Life Sciences, Eli Lilly, Incyte Corporation, Innovaderm Research, Novartis, Pfizer, Promius Pharma, Regeneron, UCB, Valeant Pharmaceuticals North America, and XBiotech. AZ has served as an investigator and/or consultant for AbbVie, Biofrontera, Dermavant, Galderma, Incyte, and UCB. YK, BA, XC, and HK are employees and shareholders of Incyte Corporation. ASP has served as an investigator, consultant, or data safety monitoring board member for AbbVie, Abeona, Apogee, Applied Pharma Research, Arcutis, Aslan, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, Johnson and Johnson, Krystal Biotech, LEO, Mitsubishi Tanabe, Nektar, Primus, Procter and Gamble, Regeneron, Sanofi, Seanergy, TWI Biotech, and UCB. ASP is an editorial board member of the American Journal of Clinical Dermatology. ASP was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Availability of Data and Materials: Incyte Corporation (Wilmington, DE, USA) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except Phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g., US, EU, JPN). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960 . Ethics Approval: This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was approved by the Advarra Institutional Review Board (Columbia, MD, USA). Consent to Participate: Written informed consent/assent was provided by all patients before enrollment. Consent for Publication: Not applicable. Code Availability: Not applicable. Author Contributions: LSG, RB, BA, HK, and ASP made substantial contributions to the conception and design of the work. LSG, RB, SF, AZ, and ASP all made substantial contributions to the acquisition of data. YK and XC made substantial contributions to the analysis of the data; all authors made substantial contributions to the interpretation of data; all authors drafted the work and revised it critically for important intellectual content, approved the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

