Isoferulic acid facilitates effective clearance of hypervirulent Klebsiella pneumoniae through targeting capsule
- PMID: 39761301
- PMCID: PMC11737856
- DOI: 10.1371/journal.ppat.1012787
Isoferulic acid facilitates effective clearance of hypervirulent Klebsiella pneumoniae through targeting capsule
Abstract
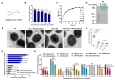
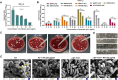
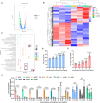
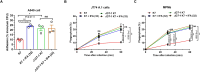
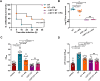
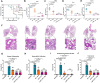
Hypervirulent Klebsiella pneumoniae (hvKP) poses an alarming threat in clinical settings and global public health owing to its high pathogenicity, epidemic success and rapid development of drug resistance, especially the emergence of carbapenem-resistant lineages (CR-hvKP). With the decline of the "last resort" antibiotic class and the decreasing efficacy of first-line antibiotics, innovative alternative therapeutics are urgently needed. Capsule, an essential virulence determinant, is a major cause of the enhanced pathogenicity of hvKP and thus represents an attractive drug target to prevent the devastating clinical outcomes caused by hvKP infection. Here, we identified isoferulic acid (IFA), a natural phenolic acid compound widely present in traditional herbal medicines, as a potent broad-spectrum K. pneumoniae capsule inhibitor that suppresses capsule polysaccharide synthesis by increasing the energy status of bacteria. In this way, IFA remarkably reduced capsule thickness and impaired hypercapsule-associated hypermucoviscosity phenotype (HMV), thereby significantly sensitizing hvKP to complement-mediated bacterial killing and accelerating host cell adhesion and phagocytosis. Consequently, IFA facilitated effective bacterial clearance and thus remarkably protected mice from lethal hvKP infection, as evidenced by limited bacterial dissemination and a significant improvement in survival rate. In conclusion, this work promotes the development of a capsule-targeted alternative therapeutic strategy for the use of the promising candidate IFA as an intervention to curb hvKP infection, particularly drug-resistant cases.
Copyright: © 2025 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Flynn CE, Guarner J. Emerging Antimicrobial Resistance. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2023;36(9):100249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

