Sequence and biochemical analysis of vaccinia virus A32 protein: Implications for in vitro stability and coiled-coil motif mediated regulation of the DNA-dependent ATPase activity
- PMID: 39761312
- PMCID: PMC11703096
- DOI: 10.1371/journal.pone.0316818
Sequence and biochemical analysis of vaccinia virus A32 protein: Implications for in vitro stability and coiled-coil motif mediated regulation of the DNA-dependent ATPase activity
Abstract
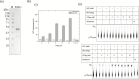
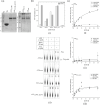
Nucleocytoplasmic large DNA viruses (NCLDVs) have massive genome and particle sizes compared to other known viruses. NCLDVs, including poxviruses, encode ATPases of the FtsK/HerA superfamily to facilitate genome encapsidation. However, their biochemical and structural characteristics are yet to be discerned. In this study, we demonstrate that the viral ATPases are significantly shorter than their bacterial homologs, representing the minimal ATPase core of the FtsK/HerA superfamily. We analysed the sequence and secondary structural features of the vaccinia virus A32 protein and determined their roles in the protein's ATPase activity. We sought to purify A32 by various techniques and noted that recombinant A32 expressed in E. coli is highly insoluble and unstable in solution. N-terminal fusion with the thioredoxin solubility tag could alleviate this issue to some extent, but subsequent tag cleavage results in increased susceptibility to precipitation and degradation. We have also predicted a highly conserved coiled-coil motif (CCM) towards the C-terminus of vaccinia virus A32. ATPase activity of A32 is known to increase in the presence of DNA. Comparative analysis of the wildtype protein versus its CCM mutants suggests that this DNA dependence of A32's ATPase activity is likely regulated by the CCM. We demonstrate that oligomerization of A32, mediated by the CCM, is required for its DNA-binding but is not dependent on ATP- or DNA-binding. Our findings suggest a key role of the CCM, and thus, higher-order structure formation in the regulated ATPase activity of A32, providing new opportunities for further detailed characterization of the poxvirus genome packaging process.
Copyright: © 2025 Ramakrishnan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging.Nucleic Acids Res. 2004 Oct 5;32(17):5260-79. doi: 10.1093/nar/gkh828. Print 2004. Nucleic Acids Res. 2004. PMID: 15466593 Free PMC article.
-
Goatpoxvirus ATPase activity is increased by dsDNA and decreased by zinc ion.Virus Genes. 2016 Oct;52(5):625-32. doi: 10.1007/s11262-016-1349-3. Epub 2016 May 5. Virus Genes. 2016. PMID: 27146321
-
Gene A32 product of vaccinia virus may be an ATPase involved in viral DNA packaging as indicated by sequence comparisons with other putative viral ATPases.Virus Genes. 1993 Feb;7(1):89-94. doi: 10.1007/BF01702351. Virus Genes. 1993. PMID: 8470370
-
Comparative genomics and evolutionary trajectories of viral ATP dependent DNA-packaging systems.Genome Dyn. 2007;3:48-65. doi: 10.1159/000107603. Genome Dyn. 2007. PMID: 18753784 Review.
-
A history of poly A sequences: from formation to factors to function.Prog Nucleic Acid Res Mol Biol. 2002;71:285-389. doi: 10.1016/s0079-6603(02)71046-5. Prog Nucleic Acid Res Mol Biol. 2002. PMID: 12102557 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

