Aging oocytes: exploring apoptosis and its impact on embryonic development in common carp (Cyprinus carpio)
- PMID: 39761344
- PMCID: PMC11757700
- DOI: 10.1093/jas/skaf002
Aging oocytes: exploring apoptosis and its impact on embryonic development in common carp (Cyprinus carpio)
Abstract
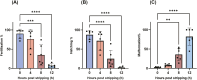
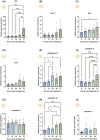
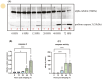
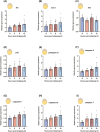
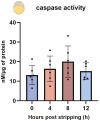
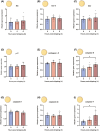
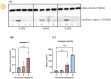
Ovulation, fertilization, and embryo development are orchestrated and synchronized processes essential for the optimal health of offspring. Postovulatory aging disrupts this synchronization and impairs oocyte quality. In addition, oocyte aging causes fertilization loss and poor embryo development. This investigation aimed to unravel the endpoint of in vitro oocyte aging in common carp (Cyprinus carpio) to understand the involvement of apoptosis in postovulatory oocyte death. It was observed that the fertilization ability significantly declined (P < 0.001) at 8-h poststripping (HPS), subsequently triggering apoptosis in the advanced stage of oocyte aging, i.e., 48 HPS. This process included an increase in proapoptotic transcripts (fas, bax, cathepsin D, caspase 8, caspase 9, and caspase 3a) (P < 0.05), elevated levels of caspase 3 protein (P < 0.05), and activation of caspase 3 enzyme (P < 0.001), a key player in apoptosis, in aging oocytes. Furthermore, the effects of oocyte aging on the embryonic apoptosis machinery were examined in embryos at 5-h postfertilization (HPF) and 24 HPF derived from fresh and aged oocytes. Expression of apoptotic genes and caspase enzyme activity remained at the basal level in 5 HPF (early blastula embryos) from both fresh and aged oocytes. In contrast, the zymogenic and active forms of caspase 3 increased in 24 HPF embryos from 8-h-aged oocytes (P < 0.01) compared with those from fresh oocytes. Thus, apoptosis intensified in 24 HPF embryos from aged oocytes without affecting the apoptotic machinery of early blastula embryos. Our findings demonstrate that apoptosis initiated by the Fas/FasL system is an important physiological process accompanying oocyte aging in common carp.
Keywords: apoptosis; caspases; early blastula embryos; fish; in-vitro-aged oocytes; spontaneous activation.
Plain language summary
The delay in fertilization after ovulation or retention of ovulated oocytes in the fish body causes postovulatory aging. Postovulatory aging leads to time-dependent deterioration of oocyte quality and loss of fertilization capacity. The mechanisms behind losing oocyte quality and developmental capacity due to postovulatory oocyte aging remain elusive. The emerging climate change issues in nature and unfavorable spawning conditions have caused the retention of ovulated oocytes in the female body. Analyzing the apoptotic parameters to understand the fate of these aged oocytes and the consequences of this aging on embryo development was the main objective of this study. The results obtained from this study indicate that aged oocytes die by apoptosis. The embryos from aged oocytes show more apoptosis, stating that oocyte aging affects embryo development by affecting the intensity of apoptosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society of Animal Science.
Conflict of interest statement
The authors declare no financial or nonfinancial interests to disclose.
Figures

Similar articles
-
Molecular mechanism of poor embryo development in postovulatory aged oocytes: mini review.J Obstet Gynaecol Res. 2013 Oct;39(10):1431-9. doi: 10.1111/jog.12111. Epub 2013 Jul 22. J Obstet Gynaecol Res. 2013. PMID: 23876057 Review.
-
[Mechanisms of the Effect of Maternal Age-Related Oocyte Aging on Fertility: Transcriptomic Sequencing Analysis of a Zebrafish Model].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):588-595. doi: 10.12182/20240560205. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948296 Free PMC article. Chinese.
-
Postovulatory aging affects dynamics of mRNA, expression and localization of maternal effect proteins, spindle integrity and pericentromeric proteins in mouse oocytes.Hum Reprod. 2016 Jan;31(1):133-49. doi: 10.1093/humrep/dev279. Epub 2015 Nov 17. Hum Reprod. 2016. PMID: 26577303 Free PMC article.
-
In vitro postovulatory oocyte aging affects H3K9 trimethylation in two-cell embryos after IVF.Ann Anat. 2020 Jan;227:151424. doi: 10.1016/j.aanat.2019.151424. Epub 2019 Oct 11. Ann Anat. 2020. PMID: 31610252
-
In vivo and in vitro postovulatory aging: when time works against oocyte quality?J Assist Reprod Genet. 2022 Apr;39(4):905-918. doi: 10.1007/s10815-022-02418-y. Epub 2022 Mar 21. J Assist Reprod Genet. 2022. PMID: 35312936 Free PMC article. Review.
References
-
- Adams, J. M., and Cory S... 1998. The Bcl-2 protein family: arbiters of cell survival. Science. 281:1322–1326. doi: https://doi.org/10.1126/science.281.5381.1322 - DOI - PubMed
-
- Aegerter, S., Jalabert B., and Bobe J... 2005. Large scale real‐time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and post‐ovulatory aging. Mol. Reprod. Dev. 72:377–385. doi: https://doi.org/10.1002/mrd.20361 - DOI - PubMed
-
- Algeciras-Schimnich, A., Shen L., Barnhart B. C., Murmann A. E., Burkhardt J. K., and Peter M. E... 2002. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 22:207–220. doi: https://doi.org/10.1128/MCB.22.1.207-220.2002 - DOI - PMC - PubMed
-
- Ashkenazi, A., and Dixit V. M... 1998. Death receptors: signaling and modulation. Science. 281:1305–1308. doi: https://doi.org/10.1126/science.281.5381.1305 - DOI - PubMed
-
- Azuma, T., Ohta H., Oda S., Muto K., Yada T., and Unuma T... 2003. Changes in fertility of rainbow trout eggs retained in coelom. Fisheries Sci. 69:131–136. doi: https://doi.org/10.1046/j.1444-2906.2003.00597.x - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

