Olfaction with legs-Spiders use wall-pore sensilla for pheromone detection
- PMID: 39761388
- PMCID: PMC11760499
- DOI: 10.1073/pnas.2415468121
Olfaction with legs-Spiders use wall-pore sensilla for pheromone detection
Erratum in
-
Correction for Talukder et al., Olfaction with legs-Spiders use wall-pore sensilla for pheromone detection.Proc Natl Acad Sci U S A. 2025 Feb 25;122(8):e2501234122. doi: 10.1073/pnas.2501234122. Epub 2025 Feb 6. Proc Natl Acad Sci U S A. 2025. PMID: 39913184 Free PMC article. No abstract available.
Abstract
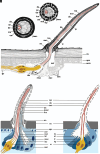
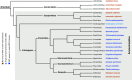
The sense of smell is a central sensory modality of most terrestrial species. However, our knowledge of olfaction is based on vertebrates and insects. In contrast, little is known about the chemosensory world of spiders and nothing about how they perform olfaction despite their important ecological role. The orb-weaving spider Argiope bruennichi lends itself to an in-depth study on olfaction as it is one of the few spider species whose volatile sex pheromone, emitted by females to attract males, is known. We combined ultrastructural and electrophysiological analyses and found that previously overlooked sensilla with wall pores are abundant on all walking legs of A. bruennichi males. We compared the ultrastructure of these wall-pore sensilla with those known to perform olfaction in insects, exploring similarities and differences. Electrophysiological single sensillum recordings demonstrated that the wall-pore sensilla in A. bruennichi respond highly sensitive and in a concentration-dependent manner to the sex pheromone. Our study demonstrates male-specific sensilla for detecting signaling females, whereas females and subadult males are devoid of wall pore sensilla. In a preliminary comparative morphological analysis using 19 species from 16 spider families, we found that wall-pore sensilla occur in male spiders from most, but not in basally branching clades or in Salticids, suggesting that wall-pore sensilla evolved at least once within spiders and were lost at least once. This research significantly expands our knowledge of the sensory ecology of spiders, will stimulate studies on the diversity and function of sensilla, as well as studies on the evolution of olfaction in arthropods.
Keywords: Araneae; chemosensing; electrophysiology; mate attraction; ultrastructure.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Zacharuk R. Y., Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 25, 27–47 (1980).
-
- Steinbrecht R. A., “Olfactory receptors” in Atlas of Arthropod Sensory Receptors. Dynamic Morphology in Relation to Function, Eguchi E., Tominaga Y., Eds. (Springer, Tokyo, 1999), pp. 155–176.
-
- Foelix R. F., “Mechano- and chemoreceptive sensilla” in Neurobiology of Arachnids, Barth F. G., Ed. (Springer, Berlin Heidelberg, 1985), pp. 118–137.
-
- Tichy H., Gingl E., Ehn R., Papke M., Schulz S., Female sex pheromone of a wandering spider (Cupiennius salei): Identification and sensory reception. J. Comp. Physiol. [A] 187, 75–78 (2001). - PubMed
-
- Ganske A.-S., Uhl G., The sensory equipment of a spider—A morphological survey of different types of sensillum in both sexes of Argiope bruennichi (Araneae, Araneidae). Arthropod Struct. Dev. 47, 144–161 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

