Datopotamab Deruxtecan in Advanced or Metastatic Non-Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study
- PMID: 39761483
- PMCID: PMC11949215
- DOI: 10.1200/JCO-24-01349
Datopotamab Deruxtecan in Advanced or Metastatic Non-Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study
Abstract
Purpose: Datopotamab deruxtecan (Dato-DXd) is a trophoblast cell-surface antigen-2-directed antibody-drug conjugate with a highly potent topoisomerase I inhibitor payload. The TROPION-Lung05 phase II trial (ClinicalTrials.gov identifier: NCT04484142) evaluated the safety and clinical activity of Dato-DXd in patients with advanced/metastatic non-small cell lung cancer (NSCLC) with actionable genomic alterations progressing on or after targeted therapy and platinum-based chemotherapy.
Patients and methods: Patients received Dato-DXd 6 mg/kg once every 3 weeks. The primary end point was objective response rate (ORR) by blinded independent central review. Secondary end points included duration of response (DOR), safety, tolerability, and survival.
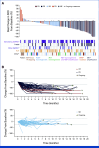
Results: Among 137 patients who received at least 1 dose of Dato-DXd, 71.5% received at least three lines of prior therapies for advanced/metastatic disease. Overall, 56.9% had EGFR mutations and 24.8% had ALK rearrangements. Median treatment duration was 4.4 months (range, 0.7-20.6). The confirmed ORR was 35.8% (95% CI, 27.8 to 44.4) overall, and 43.6% (95% CI, 32.4 to 55.3) and 23.5% (95% CI, 10.7 to 41.2) in those with EGFR mutations and ALK rearrangements, respectively. The median DOR was 7.0 months (95% CI, 4.2 to 9.8), and the overall disease control rate was 78.8% (95% CI, 71.0 to 85.3). Grade ≥3 treatment-related adverse events (TRAEs) occurred in 28.5% of patients. The most common TRAE was stomatitis (preferred term; any grade: 56.2%; grade ≥3: 9.5%). Five (3.6%) patients experienced adjudicated treatment-related interstitial lung disease/pneumonitis, with 1 (0.7%) grade 5 event.
Conclusion: Encouraging and durable antitumor activity was observed with Dato-DXd in this heavily pretreated advanced/metastatic NSCLC population with actionable genomic alterations. The rate of treatment-related grade ≥3 toxicities was comparable with previous observations, and no new safety signals were observed.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 - PubMed
-
- Hendriks LE, Kerr KM, Menis J, et al. : Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:339-357, 2023 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

