Plasma pTau181 and amyloid markers predict conversion to dementia in idiopathic REM sleep behaviour disorder
- PMID: 39761530
- PMCID: PMC12129744
- DOI: 10.1093/brain/awaf003
Plasma pTau181 and amyloid markers predict conversion to dementia in idiopathic REM sleep behaviour disorder
Abstract
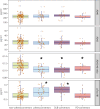
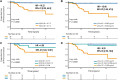
Blood-based biomarkers for Alzheimer's disease (AD) pathology have been investigated intensively as markers for AD-related neurodegeneration. Comorbid AD pathology is common in dementia with Lewy bodies (DLB). Accordingly, we hypothesized that plasma biomarkers associated with AD pathology might be useful to predict DLB in a cohort of idiopathic/isolated REM sleep behaviour disorder (iRBD), an incipient synucleinopathy. The aim of this study was to determine whether plasma amyloid-β and pTau181 biomarkers can predict DLB. This longitudinal single-centre (Canada) cohort study included 158 individuals with polysomnography-confirmed iRBD between September 2004 and October 2022, each providing blood plasma samples, who were then offered prospective follow-up. Plasma Aβ40, Aβ42 and pTau181 levels were measured using NeuroToolKit, a prototype assay panel of neurodegeneration (Roche Diagnostics International Ltd). The primary outcome was the association between plasma biomarkers at baseline and eventual development of DLB. Correlations between plasma markers and baseline cognitive tests were assessed. A total of 142 iRBD participants [109 male (77%); mean ± SD, 67.6 ± 8.0 years of age] were included in the final analysis. On prospective follow-up (2.9 ± 2.1 years after sampling), 32 individuals phenoconverted to a defined neurodegenerative syndrome (18 DLB, 13 Parkinson's disease and one multiple system atrophy). The combined phenoconvertor group had lower baseline plasma Aβ42/40 ratio compared with non-phenoconvertors (mean ± SD, 0.103 ± 0.010 versus 0.114 ± 0.012, P < 0.001) and higher pTau181 levels (0.993 ± 0.354 versus 0.784 ± 0.266 pg/ml, P = 0.008). When divided by phenoconversion subtype, significant differences were seen selectively in DLB convertors [Aβ42/40 = 0.101 ± 0.010, difference -0.011, 95% confidence interval (-0.016; -0.005), P < 0.001; pTau181 = 1.144 ± 0.326 pg/ml, difference 0.282 pg/ml, 95% confidence interval (0.146; 0.418), P < 0.001]. Cross-sectional analysis showed that plasma pTau181 (but not Aβ42/40) was correlated with cognitive tests across various domains. Our results indicate that plasma Aβ42/40 ratio and pTau181 can predict conversion to DLB in iRBD.
Keywords: Aβ42; blood markers; dementia with Lewy bodies; idiopathic/isolated REM sleep behaviour disorder; pTau181.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The NeuroToolKit is a panel of exploratory prototype assays designed to robustly evaluate biomarkers associated with key pathological events characteristic of AD and other neurological disorders, used for research purposes only and not approved for clinical use (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). COBAS and ELECSYS are trademarks of Roche. All other product names and trademarks are the property of their respective owners. A.D., A.P. and E.S. have no competing interests. J.-F.G. reports grants from the FRQ-S, CIHR, W. Garfield Weston Foundation, and NIH/National Institute on Aging and holds a Canada Research Chair in Cognitive Decline in Pathological Aging. J.M. reports grants from Canadian Institute of Health Research. G.K. is a full-time employee of Roche Diagnostics GmbH. T.K.T., T.K. and V.M. are full-time employees of Roche Pharma Research and Early Development and shareholders of F. Hoffmann-La Roche Ltd. Z.G.-O. is supported by the Fonds de recherche du Québec—Santé (FRQS) Chercheurs-boursiers award, and is a William Dawson Scholar, and received consultancy fees from Idorsia, Prevail Therapeutics, Ono Therapeutics, Denali, Handl Therapeutics, Neuron23, Bial Biotech, Bial, UCB, Capsida, Vanquabio, Jazz Pharmaceuticals and Takeda. R.B.P. is supported by the National Institutes of Health, the Michael J. Fox Foundation and the Webster Foundation and reports personal fees from Takeda, Biogen, Abbvie, Curasen, Lilly, Novartis, Eisai, Paladin, Merck, Vaxxinity, Korro, Bristol Myers Squibb and the International Parkinson and Movement Disorders Society, outside the submitted work.
Figures

Comment in
-
Markers help to predict dementia with Lewy bodies.Nat Rev Neurol. 2025 Feb;21(2):66. doi: 10.1038/s41582-025-01058-x. Nat Rev Neurol. 2025. PMID: 39820144 No abstract available.
References
-
- Schoonenboom NS, Reesink FE, Verwey NA, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. - PubMed
-
- Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–386. - PubMed

