Low versus high initial oral glucocorticoid dose for lupus nephritis: a pooled analysis of randomised controlled clinical trials
- PMID: 39762088
- PMCID: PMC11752037
- DOI: 10.1136/lupus-2024-001351
Low versus high initial oral glucocorticoid dose for lupus nephritis: a pooled analysis of randomised controlled clinical trials
Abstract
Objective: Traditional initial treatment regimens for lupus nephritis (LN) used oral glucocorticoids (GC) in starting doses up to 1.0 mg/kg/day prednisone equivalent with or without a preceding intravenous methylprednisolone pulse. More recent management guidelines recommend lower starting oral GC doses following intravenous pulse therapy. As there have been no large studies directly comparing patients receiving low versus high initial oral GC doses, this pooled analysis of high-quality randomised controlled trials (RCTs) aims to evaluate differences in efficacy and safety.
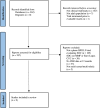
Methods: Published data were analysed from RCTs that assessed variable GC doses in the standard of care (SOC) treatment arms. Patients receiving starting prednisone doses up to 0.5 mg/kg/day (low dose) were compared with 1.0 mg/kg/day (high dose). Complete renal response requiring urine protein-creatinine ratio <0.5 mg/mg (CRR 0.5), CRR or partial renal response (PRR), serious adverse events (SAE) and SAE due to infections at 12 months of treatment were compared between groups.
Results: 417 patients from SOC arms of five studies were exposed to low-dose initial GC after intravenous pulse, while 521 patients from four studies were treated with high-dose oral GC. In patients with low-dose oral GC, 25.2% achieved CRR 0.5 at 12 months compared with 27.2% in high-dose groups, p=0.54. CRR or PRR was attained in 48.7% low-dose vs 43.6% high-dose patients, p=0.14. SAEs and infection SAEs were less common in the low-dose GC group (19.4% vs 31.6%, p<0.001 and 9.8% vs 16.5%, p=0.012, respectively).
Conclusions: Based on pooled RCT data, there was no significant difference in 12-month renal responses between patients receiving low-dose prednisone following intravenous GC compared with those receiving initial high doses. SAEs were less frequent in patients receiving low-dose initial GC. These findings support the use of lower oral GC doses in LN treatment.
Keywords: clinical trial; lupus erythematosus, systemic; lupus nephritis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AS, consultant: AbbVie, Amgen, AstraZeneca, Aurinia, BMS, Eli Lilly, GlaxoSmithKline, Synthekine; PI, consultant: GlaxoSmithKline, Momenta/Janssen; HMB, consultant: Aurinia, Alexion, Vertex Pharmaceuticals; JPB, consultant: Bristol-Myers Squibb; GlaxoSmithKline, Related Sciences, Ventus Therapeutics.
Figures
References
-
- Apostolopoulos D, Kandane-Rathnayake R, Louthrenoo W, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus with no clinical or serological disease activity: a multicentre cohort study. Lancet Rheumatol. 2020;2:e24–30. doi: 10.1016/S2665-9913(19)30105-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous