Evaluation of rheumatoid arthritis-associated interstitial lung disease in patients treated with JAK inhibitors: a MAJIK-SFR cohort study
- PMID: 39762124
- PMCID: PMC11749054
- DOI: 10.1136/rmdopen-2024-005062
Evaluation of rheumatoid arthritis-associated interstitial lung disease in patients treated with JAK inhibitors: a MAJIK-SFR cohort study
Abstract
Objective: To examine the course of interstitial lung disease associated with rheumatoid arthritis (RA-ILD) in France on treatment with Janus kinase inhibitors (JAKis) using the MAJIK-SFR registry.
Methods: Prospective national multicentre observational study identifying patients with RA-ILD from the MAJIK-SFR registry. Pulmonary assessment data were collected at JAKi initiation and follow-up visits (6 months, 12 months and a median of 21 months postinclusion), including chest high-resolution CT (HRCT), pulmonary function tests (forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO)), acute exacerbations of ILD, respiratory infections and lung cancers.
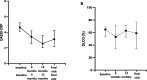
Results: We enrolled 42 patients (26 women, 62%) with RA-ILD with a mean age of 61±13 years and a mean disease duration of 16±10 years. Compared with the 778 RA patients without ILD from the MAJIK registry, RA-ILD patients were older, displayed more severe and active disease and had more prevalent comorbidities. Non-specific interstitial pneumonia and usual interstitial pneumonia accounted for 46% and 43% of the chest HRCT ILD patterns, respectively. No significant changes in FVC and DLCO were observed during the follow-up period. Chest HRCT lesions remained stable in 69% of patients. Progressive ILD was identified in 8 patients (19%). 16 (38%) respiratory tract infections were observed. Only one acute regressive exacerbation of ILD was noted, and no lung cancer was diagnosed. No deaths occurred. JAKi was discontinued in 17 patients including 8 for inefficacy on joint involvement and 5 for intolerance.
Conclusion: The analysis indicates stability of RA-ILD in patients treated with JAKi. The tolerance profile of JAKi in this higher risk population did not reveal new safety signal.
Keywords: Biological Therapy; Pulmonary Fibrosis; Rheumatoid Arthritis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JA: Honoraria: Galapagos, Alfasigma, Lilly, Pfizer, Abbvie, Bristol-Myers Squibb, Sanofi, Roche-Chugai, Nordic Pharma, Medac, Novartis, Biogen, Fresenius Kabi, Janssen and MSD. Research grants: Bristol-Myers Squibb, Pfizer (Passerelle), Novartis (Dreamer), Fresenius Kabi, Galapagos/Alfasigma, Nordic Pharma. M-ET and CP: Honoraria: Galapagos, Alfasigma, Lilly, Pfizer, Abbvie.
Figures
References
-
- Salaffi F, De Angelis R, Grassi W, et al. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol . 2005;23:819–28. - PubMed
-
- Salaffi F, Carotti M, Di Carlo M, et al. High-resolution computed tomography of the lung in patients with rheumatoid arthritis: Prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine (Baltimore) 2019;98:e17088. doi: 10.1097/MD.0000000000017088. - DOI - PMC - PubMed
-
- Spagnolo P, Lee JS, Sverzellati N, et al. The Lung in Rheumatoid Arthritis: Focus on Interstitial Lung Disease. Arthritis Rheumatol. 2018;70:1544–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
