Outcomes and predictive factors for fluid resolution following three loading injections of faricimab for treatment-naïve neovascular age-related macular degeneration
- PMID: 39762260
- PMCID: PMC11704234
- DOI: 10.1038/s41598-024-82746-4
Outcomes and predictive factors for fluid resolution following three loading injections of faricimab for treatment-naïve neovascular age-related macular degeneration
Abstract
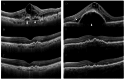
To evaluate the outcomes and predictive factors for fluid resolution following three loading injections of faricimab for neovascular age-related macular degeneration(AMD). This retrospective study included patients diagnosed with treatment-naïve neovascular AMD who received three monthly injections of faricimab. Changes in best-corrected visual acuity(BCVA) and central retinal thickness(CRT) following treatment were evaluated. The resolution of subretinal fluid(SRF), intraretinal fluid(IRF), and serous pigment epithelial detachment(PED) was also assessed. In addition, factors associated with complete resolution of SRF and IRF were investigated. A total of 69 patients were included in this study. BCVA significantly improved from a mean logarithm of minimal angle of resolution of 0.64 ± 0.41 at baseline to 0.47 ± 0.39 at 3 months (P < 0.001). CRT significantly decreased from 424.1 ± 155.5 μm at baseline to 266.3 ± 71.7 μm at 3 months (P < 0.001). At baseline, SRF was observed in 55 eyes (79.7%), IRF in 39 eyes(56.5%), and serous PED in 57 eyes(82.6%). By 3 months, the number of eyes showing these findings had decreased to 11 eyes(15.9%) for SRF, 6 eyes(8.7%) for IRF, and 10 eyes(14.5%) for serous PED. The presence of type 2 (88.2%) and type 3 (94.7%) macular neovascularization(MNV) was associated with a high incidence of complete resolution of SRF and IRF after treatment. Three loading injections of faricimab resulted in significant functional and anatomical improvements in treatment-naïve neovascular AMD, with a high rate of resolution of SRF, IRF, and serous PED. The anatomical effects were especially pronounced in cases of type 2 and type 3 MNV.
Keywords: Age-related macular degeneration; Choroidal neovascularization; Faricimab; Macular neovascularization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Jae Hui Kim received lecture fees from Bayer, Novartis, Roche, Samsung Bioepis, and Chong Kun Dang Pharmaceutical Corp. Han Joo Cho received lecture fees from Bayer, Novartis, Roche. The remaining authors (H.Y.H., S.M.P., J.H.L., C.G.K., J.W.K.) declare no conflicts of interest.
Figures



References
-
- Shirley, M. & Faricimab First Approval Drugs ; 82: 825–830. (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

