Ferric carboxymaltose for anemia in late pregnancy: a randomized controlled trial
- PMID: 39762420
- PMCID: PMC11750709
- DOI: 10.1038/s41591-024-03385-w
Ferric carboxymaltose for anemia in late pregnancy: a randomized controlled trial
Erratum in
-
Author Correction: Ferric carboxymaltose for anemia in late pregnancy: a randomized controlled trial.Nat Med. 2025 Jan;31(1):351. doi: 10.1038/s41591-025-03492-2. Nat Med. 2025. PMID: 39806073 Free PMC article. No abstract available.
Abstract
Over 46% of African pregnant women are anemic. Oral iron is recommended but often suboptimal, particularly late in pregnancy. Intravenous ferric carboxymaltose (FCM) could treat anemia in women in the third trimester in sub-Saharan Africa. In an open-label, individually randomized trial in antenatal clinics in southern Malawi, we randomized 590 women at 27-35 weeks of gestation with capillary hemoglobin <10.0 g dl-1 to FCM (20 mg kg-1 up to 1,000 mg, once at enrollment) or standard of care (60 mg elemental iron, twice daily for 90 days). Participants and their infants were followed to 4 weeks postpartum. Primary outcomes were maternal anemia at 36 weeks' gestation or delivery (whichever occurred first) and neonatal birthweight. At the primary timepoint, 126 of 270 (46.7%) of women in the FCM group were anemic, compared to 170 of 271 (67.3%) women in the standard-of-care group (PR, 0.74 (95% CI 0.64, 0.87); P = 0.0002). There was no difference between groups in birthweight (mean difference 10.9 g (-65.7, 87.5 g); P = 0.78). No serious infusion-related reactions occurred, and there were no differences in adverse events between groups. In Malawian women in late pregnancy, FCM effectively and safely reduced anemia before childbirth. Australia New Zealand Clinical Trial registration: ANZCTR12621001239853.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.-R.P. reports advisory boards and consultancy for CSL-Vifor, consultancies for ITL Biomedical and Givewell and noncompensated roles as Director of the WHO Collaborating Centre for Anemia Detection and Control. The other authors have no competing interests.
Figures
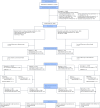

References
-
- World Health Organisation. Global Health Observatory: Prevalence of Anaemia in Pregnant Women (aged 15-49) (%) (World Health Organization, 2024).
-
- World Health Organization. The Global Prevalence of Anaemia in 2011 (WHO, 2015).
-
- World Health Organization. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women (WHO, 2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

