Selumetinib in adults with NF1 and inoperable plexiform neurofibroma: a phase 2 trial
- PMID: 39762421
- PMCID: PMC12423960
- DOI: 10.1038/s41591-024-03361-4
Selumetinib in adults with NF1 and inoperable plexiform neurofibroma: a phase 2 trial
Abstract
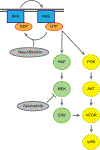
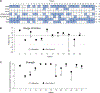
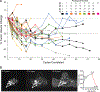
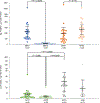
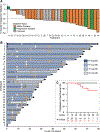
The MEK inhibitor selumetinib induces objective responses and provides clinical benefit in children with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs). To evaluate whether similar outcomes were possible in adult patients, in whom PN growth is generally slower than in pediatric patients, we conducted an open-label phase 2 study of selumetinib in adults with NF1 PNs. The study was designed to evaluate objective response rate (primary objective), tumor volumetric responses, patient-reported outcomes and pharmacodynamic effects in PN biopsies. The objective response rate was 63.6% (21/33 participants). Median maximal PN volume decrease was 23.6% (range: -48.1% to 5.5%). No disease progression relative to baseline PN volumes occurred before data cutoff, with a median of 28 cycles completed (range: 1-78, 28 d per cycle). Participants experienced decreased tumor pain intensity and pain interference. Adverse events (AEs) were similar to those of the pediatric trial; acneiform rash was the most prevalent AE. Phosphorylation ratios of ERK1/2 decreased significantly (ERK1 median change: -64.6% (range: -99.5% to 90.7%), ERK2 median change: -57.3% (range: -99.9% to 84.4%)) in paired PN biopsies (P ≤ 0.001 for both isoforms) without compensatory phosphorylation of AKT1/2/3. The sustained PN volume decreases, associated improvement in pain and manageable AE profile indicate that selumetinib provides benefit to adults with NF1 and inoperable PNs. ClinicalTrials.gov identifier: NCT02407405 .
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE & Mautner VF Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. J Pediatr 159, 652–655 e652 (2011). - PubMed
-
- Canavese F & Krajbich JI Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J Pediatr Orthop 31, 303–311 (2011). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

