Once daily Bromelain-based enzymatic debridement of venous leg ulcers versus gel vehicle (placebo) and non-surgical standard of care: a three-arm multicenter, double blinded, randomized controlled study
- PMID: 39763591
- PMCID: PMC11701486
- DOI: 10.1016/j.eclinm.2024.102750
Once daily Bromelain-based enzymatic debridement of venous leg ulcers versus gel vehicle (placebo) and non-surgical standard of care: a three-arm multicenter, double blinded, randomized controlled study
Abstract
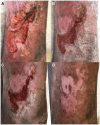
Background: Debridement is considered the first step in treatment of chronic wounds, however, current enzymatic and autolytic debridement agents are slow or ineffective. Previous studies have shown positive initial results with EscharEx® (EX-02 formulation), a Bromelain-based enzymatic debridement agent in development for chronic wounds. The main objective of this study was to assess its efficacy in debriding venous leg ulcers (VLU), compared to gel vehicle (GV) as a placebo control and to non-surgical standard of care (NSSOC).
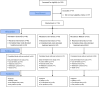
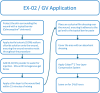
Methods: A prospective, randomized, multicenter, placebo-controlled trial in patients with VLU from 20 medical centers and clinics in the United States, Switzerland and Israel was undertaken. Patients were treated with daily topical applications of either EX-02, GV, or NSSOC (in a 3:3:2 ratio), until reaching complete debridement or up to 8 daily treatments (within 2 weeks), and then followed-up for up to 14 weeks. The primary efficacy endpoint was the incidence of complete debridement. This study is registered with ClinicalTrials.gov (NCT03588130) and EudraCT (number 2020-004861-38).
Findings: A total of 196 patients were enrolled, and 119 randomized (between November 12th, 2019, and February 15th, 2022); 46 to the EX-02 arm, 43 to the GV arm, and 30 to the NSSOC arm. Eight patients dropped out of the study (2 in EX-02, 2 in GV, 4 in NSSOC). The incidence of complete debridement within 8 daily treatments was 63% (29/46 patients) in the EX-02 arm as compared to 30.2% (13/43 patients) in the GV arm (p = 0.004) and 13.3% (4/30 patients) in the NSSOC arm (p < 0.001). Sixty-five patients reported wound related adverse events throughout the study; 24 (52.2%), 27 (62.8%) and 14 (46.7%) patients in the EX-02, GV and NSSOC arms (p = NS). No deaths occurred during the study.
Interpretation: EX-02 lead to a significantly higher incidence of complete debridement as compared to GV and NSSOC, without significant safety issues. Additional studies are needed to explore the benefits of EX-02 in VLU and other chronic wound etiologies.
Funding: MediWound Ltd.
Keywords: Chronic wounds; Enzymatic debridement; EscharEx; RCT; VLU; Venous leg ulcer.
© 2024 The Authors.
Conflict of interest statement
YS is a consultant for MediWound Ltd and Vericel Corp. LR, JCL and CD are consultants for MediWound Ltd. RJS, EK, KDZ, MK and YKL are MedWound's Chief Medical Officer, Chief R&D Officer, Director of Clinical Affairs, Medical Director and Director of Clinical Development. AR served as an advisory board member for MediWound Ltd. AR and LTT report receiving study funding. The other authors report no competing interests beyond funding to their institutions for the current project.
Figures



References
-
- Tatsioni A., Balk E., O'Donnell T., Lau J. Usual care in the management of chronic wounds: a review of the recent literature. J Am Coll Surg. 2007;205(4):617–24e57. - PubMed
-
- O'Donnell T.F., Jr., Passman M.A., Marston W.A., et al. Management of venous leg ulcers: clinical practice guidelines of the society for vascular surgery ® and the American venous forum. J Vasc Surg. 2014;60(2 Suppl):3s–59s. - PubMed
-
- Wicke C., Bachinger A., Coerper S., Beckert S., Witte M.B., Königsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized wound care center. Wound Repair Regen. 2009;17(1):25–33. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

