Detection of aberrant locomotor activity in a mouse model of lung cancer via home cage monitoring
- PMID: 39763604
- PMCID: PMC11701213
- DOI: 10.3389/fonc.2024.1504938
Detection of aberrant locomotor activity in a mouse model of lung cancer via home cage monitoring
Abstract
Introduction: Lung cancer is the first cause of cancer death in the world, due to a delayed diagnosis and the absence of efficacy therapies. KRAS mutation occurs in 25% of all lung cancers and the concomitant mutations in LKB1 determine aggressive subtypes of these tumors. The improvement of therapeutical options for KRASG12C mutations has increased the possibility of treating these tumors, but resistance to these therapies has emerged. Preclinical animal models permit the study of tumors and the development of new therapies. The DVC system was used to measure circadian activity changes indicative of lung cancer progression in KRAS and KRAS-LKB1 transgenic mouse models.
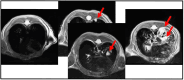
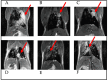
Material and methods: KRAS and KRAS-LKB1 conditional transgenic animal models were bred and genotyped. The tumors were inducted using adeno-CRE-recombinase system. The mice were housed in a Digital Ventilated Cage (DVC®) rack measuring the locomotor activity continuously for 24/7. The progression of the tumors was monitored with MRI. The DVC system evaluated a reduction in animal locomotion during the tumor progression.
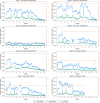
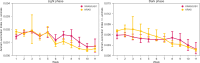
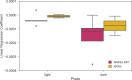
Results: KRAS and KRAS-LKB1 mutations were induced, and the tumor formation and progression were monitored over time. As expected, the onset of the tumors in the two different breeds occurred at different times. DVC system registered the locomotion activity of the mice during the light and dark phases, reporting a strong reduction, mainly, in the dark phase. In KRAS-LKB1 models, the locomotion reduction appeared more pronounced than in KRAS models.
Discussions: Transgenic animal models represent a fundamental tool to study the biology of cancers and the development of new therapies. The tumors induced in these models harbor the same genotypical and phenotypical characteristics as their human counterparts. DVC methods permit a home cage monitoring system useful for tracking animal behavior continuously 24 hours a day, 7 days a week. DVC system could determine disease progression by monitoring a single animal activity in a cage and also using group-housed animals. For these reasons, the DVC system could play a crucial role in identifying diseases at early stages and in testing new therapeutic approaches with a higher likelihood of efficacy.
Keywords: KRAS/LKB1; MRI; NSCLC; biomarker; home cage monitoring; locomotion; transgenic animal models; translational models.
Copyright © 2024 Tomanelli, Guffanti, Vargiu, Micotti, Rigamonti, Tumiatti, Caiola, Marabese and Broggini.
Conflict of interest statement
MR is the lead data scientist at Tecniplast SpA. Tecniplast SpA did not have any decisive role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. (2019) 5:1749–68. doi: 10.1001/jamaoncol.2019.2996 - DOI - PMC - PubMed
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

