Contributions of connectional pathways to shaping Alzheimer's disease pathologies
- PMID: 39763634
- PMCID: PMC11702304
- DOI: 10.1093/braincomms/fcae459
Contributions of connectional pathways to shaping Alzheimer's disease pathologies
Abstract
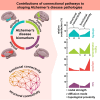
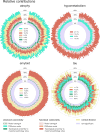
Four important imaging biomarkers of Alzheimer's disease, namely grey matter atrophy, glucose hypometabolism and amyloid-β and tau deposition, follow stereotypical spatial distributions shaped by the brain network of structural and functional connections. In this case-control study, we combined several predictors reflecting various possible mechanisms of spreading through structural and functional pathways to predict the topography of the four biomarkers in amyloid-positive patients while controlling for the effect of spatial distance along the cortex. For each biomarker, we quantified the relative contribution of each predictor to the variance explained by the model. We also compared the contribution between apolipoprotein E-ɛ4 carriers and non-carriers. We found that topological proximity to areas of maximal pathology through the functional connectome explained significant parts of variance for all biomarkers and that functional pathways totalized more than 30% of contributions for hypometabolism and amyloid load. By contrast, atrophy and tau load were mainly predicted by structural pathways, with major contributions from inter-regional diffusion. The ɛ4 allele modulated contributions to the four biomarkers in a way consistent with compromised brain connectomics in carriers. Our approach can be used to assess the contribution of concurrent mechanisms in other neurodegenerative diseases and the possible modifying impact of relevant factors on this contribution.
Keywords: Alzheimer’s disease; functional connectivity; pathology spreading; relative importance analysis; structural connectivity.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: Potential role in system degenerations, including Alzheimer’s disease. Neuroscience. 1987;23(2):389–398. - PubMed
LinkOut - more resources
Full Text Sources
