Pseudomonas plecoglossicida infection induces neutrophil autophagy-driven NETosis in large yellow croaker Larimichthys crocea
- PMID: 39763642
- PMCID: PMC11701331
- DOI: 10.3389/fimmu.2024.1521080
Pseudomonas plecoglossicida infection induces neutrophil autophagy-driven NETosis in large yellow croaker Larimichthys crocea
Abstract
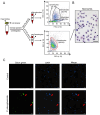
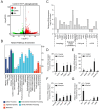
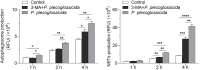
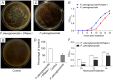
Neutrophil extracellular traps (NETs) are crucial for the immune defense of many organisms, serving as a potent mechanism for neutrophils to capture and eliminate extracellular pathogens. While NETosis and its antimicrobial mechanisms have been well studied in mammals, research on NETs formation in teleost fish remains limited. In this study, we used the large yellow croaker (Larimichthys crocea) as the study model to investigate NETosis and its role in pathogen defense. Our results showed that infection with Pseudomonas plecoglossicida could induce NETosis. To further explore the underlying mechanism, we performed transcriptome analysis and western blotting, which revealed that P. plecoglossicida triggers NETosis through activation of the autophagy pathway. Inhibition of autophagy significantly reduced NET production, highlighting its critical role in this process. Furthermore, our studies demonstrated that NETs exert a bacteriostatic effect, significantly suppressing the growth of P. plecoglossicida. Taken together, our findings reveal that autophagy regulates NETosis in large yellow croaker and underscore the essential role of NETs in bacterial defense, providing new insights into immune responses in teleost fish.
Keywords: Larimichthys crocea; Pseudomonas plecoglossicida; antibacterial; autophagy; neutrophil extracellular traps.
Copyright © 2024 Cao and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

