Metabolic adaptation of myeloid cells in the glioblastoma microenvironment
- PMID: 39763643
- PMCID: PMC11700814
- DOI: 10.3389/fimmu.2024.1431112
Metabolic adaptation of myeloid cells in the glioblastoma microenvironment
Abstract
In recent decades, immunometabolism in cancers has emerged as an interesting target for treatment development. Indeed, the tumor microenvironment (TME) unique characteristics such as hypoxia and limitation of nutrients availability lead to a switch in metabolic pathways in both tumor and TME cells in order to support their adaptation and grow. Glioblastoma (GBM), the most frequent and aggressive primary brain tumor in adults, has been extensively studied in multiple aspects regarding its immune population, but research focused on immunometabolism remains limited. Here, we provide an overview of immunometabolism adaptation of myeloid cells in cancers with a specific focus on GBM and other brain tumors, before describing current therapeutic strategies targeting metabolic pathways. The main myeloid cells composing the GBM TME include tumor-associated macrophages (TAMs), which comprise both peripheral macrophages and local microglia, as well as myeloid-derived suppressor cells. The metabolic pathways involved in myeloid cell remodeling encompass the tricarboxylic acid cycle (TCA cycle), the lipid, glucose and amino acid metabolism and hypoxia. Developing treatments that target these metabolic pathways in tumor growth and its TME is a promising and increasing field. It includes both drug-repurposing and the development of innovative metabolic therapies. We finally provide an overview of all clinical trials in neuro-oncology involving treatments modifying cell metabolism and provide the preclinical rationale for both drugs already evaluated within clinical trials and potential candidates for future trials.
Keywords: TCA cycle; glioblastoma; glycolysis; lipid metabolism; metabolism; myeloid cells.
Copyright © 2024 Essakhi, Bertucci, Baeza-Kallee, Colin, Lavignolle-Heguy, Garcia-Gonzalez, Argüello, Tchoghandjian and Tabouret.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
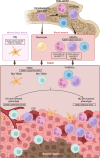
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

