Effects of chemoradiotherapy on surface PD-L1 expression in esophageal cancer and its implications for immunotherapy
- PMID: 39763660
- PMCID: PMC11701229
- DOI: 10.3389/fimmu.2024.1509051
Effects of chemoradiotherapy on surface PD-L1 expression in esophageal cancer and its implications for immunotherapy
Abstract
Background: Esophageal cancer has a poor prognosis despite treatment advancements. Although the benefit of neoadjuvant chemoradiotherapy (CRT) followed by adjuvant immunotherapy is evident, the effects of CRT on PD-L1 expression in esophageal cancer are not well understood. This study examines the impact of neoadjuvant CRT on PD-L1 surface expression in esophageal cancer both in vitro and in vivo considering its implications for immunotherapy.
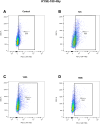
Methods: PD-L1 expression dynamics were assessed in esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) cell lines (OE-33, FLO-1, KYSE-180) treated with Carboplatin, Paclitaxel, radiotherapy (RT), and CRT. PD-L1 expression was measured by flow cytometry at 48- and 72 hours post-treatment. Temporal changes of surface PD-L1 were further investigated in KYSE-180 cells following RT, up to 168h after treatment. Additionally, PD-L1 expression was analyzed via immunohistochemistry in histological samples from 19 patients (9 EAC, 10 ESCC) treated with neoadjuvant CRT according to the CROSS-scheme.
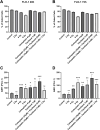
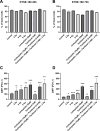
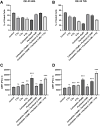
Results: PD-L1 expression was upregulated the most by Carboplatin, a combination of chemotherapy, or CRT in all cell lines. Higher irradiation doses were more effective in inducing PD-L1 expression, while Paclitaxel alone did not consistently increase PD-L1. The ESCC cell line KYSE-180 showed the highest relative PD-L1 increase. Measurement of PD-L1 kinetics revealed a transient upregulation of surface PD-L1, which peaked at 72 hours post-treatment and subsequently returned to baseline levels by 168 hours. In vivo, data demonstrated no significant PD-L1 expression changes when comparing pre- and post-treatment levels.
Conclusions: Chemotherapy, RT, and CRT can induce PD-L1 expression in various esophageal cancer cell lines. However, neoadjuvant CRT according to the CROSS protocol does not significantly induce PD-L1 in vivo. Considering the difference in time between pre- and post-therapeutic measurements, these findings suggest that PD-L1 upregulation due to neoadjuvant therapy may be transient in vivo as well. This highlights the potential benefit of administering immunotherapy in a neoadjuvant setting.
Keywords: CROSS; checkmate-577; esophageal cancer; immunotherapy; programmed-death-ligand-1.
Copyright © 2024 Hampe, Küffer, Niemeier, Scheele, Hampe, Riedl, Fischer, Ziegler, Leu, Dröge, König, Ghadimi, Braulke, Rieken, Bohnenberger and El Shafie.
Conflict of interest statement
The authors declare that the research was carried out without any commercial or financial relationships that could be interpreted as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

