This is a preprint.
Molecular and spatial analysis of ganglion cells on retinal flatmounts: diversity, topography, and perivascularity
- PMID: 39763751
- PMCID: PMC11702564
- DOI: 10.1101/2024.12.15.628587
Molecular and spatial analysis of ganglion cells on retinal flatmounts: diversity, topography, and perivascularity
Update in
-
Molecular and spatial analysis of ganglion cells on retinal flatmounts identifies perivascular neurons resilient to glaucoma.Neuron. 2025 Oct 15;113(20):3390-3407.e8. doi: 10.1016/j.neuron.2025.07.025. Epub 2025 Aug 20. Neuron. 2025. PMID: 40840447
Abstract
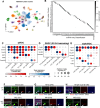
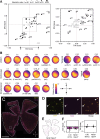
Diverse retinal ganglion cells (RGCs) transmit distinct visual features from the eye to the brain. Recent studies have categorized RGCs into 45 types in mice based on transcriptomic profiles, showing strong alignment with morphological and electrophysiological properties. However, little is known about how these types are spatially arranged on the two-dimensional retinal surface-an organization that influences visual encoding-and how their local microenvironments impact development and neurodegenerative responses. To address this gap, we optimized a workflow combining imaging-based spatial transcriptomics (MERFISH) and immunohistochemical co-staining on thin flatmount retinal sections. We used computational methods to register en face somata distributions of all molecularly defined RGC types. More than 75% (34/45) of types exhibited non-uniform distributions, likely reflecting adaptations of the retina's anatomy to the animal's visual environment. By analyzing the local neighborhoods of each cell, we identified perivascular RGCs located near blood vessels. Seven RGC types are enriched in the perivascular niche, including members of intrinsically photosensitive RGC (ipRGC) and direction-selective RGC (DSGC) subclasses. Orthologous human RGC counterparts of perivascular types - Melanopsin-enriched ipRGCs and ON DSGCs - were also proximal to blood vessels, suggesting their perivascularity may be evolutionarily conserved. Following optic nerve crush in mice, the perivascular M1-ipRGCs and ON DSGCs showed preferential survival, suggesting that proximity to blood vessels may render cell-extrinsic neuroprotection to RGCs through an mTOR-independent mechanism. Overall, our work offers a resource characterizing the spatial profiles of RGC types, enabling future studies of retinal development, physiology, and neurodegeneration at individual neuron type resolution across the two-dimensional space.
Keywords: MERFISH; neuronal types; neuroprotection; perivascular neurons; retinal ganglion cells; spatial transcriptomics.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no conflicts of interest.
Figures



References
-
- Ahnelt P.K., Fernandez E., Martinez O., Bolea J.A., and Kubber-Heiss A.. 2000. Irregular S-cone mosaics in felid retinas. Spatial interaction with axonless horizontal cells, revealed by cross correlation. J Opt Soc Am A Opt Image Sci Vis. 17:580–588. - PubMed
-
- Bae J.A., Mu S., Kim J.S., Turner N.L., Tartavull I., Kemnitz N., Jordan C.S., Norton A.D., Silversmith W.M., Prentki R., Sorek M., David C., Jones D.L., Bland D., Sterling A.L.R., Park J., Briggman K.L., Seung H.S., and Eyewirers. 2018. Digital Museum of Retinal Ganglion Cells with Dense Anatomy and Physiology. Cell. 173:1293–1306 e1219. - PMC - PubMed
Publication types
Grants and funding
- K01 MH123757/MH/NIMH NIH HHS/United States
- R01 EY036071/EY/NEI NIH HHS/United States
- R01 EY034089/EY/NEI NIH HHS/United States
- F30 EY033201/EY/NEI NIH HHS/United States
- R01 EY023648/EY/NEI NIH HHS/United States
- P30 EY012196/EY/NEI NIH HHS/United States
- R01 NS123912/NS/NINDS NIH HHS/United States
- R01 EY030138/EY/NEI NIH HHS/United States
- R01 EY027202/EY/NEI NIH HHS/United States
- R01 EY032564/EY/NEI NIH HHS/United States
- P30 EY002162/EY/NEI NIH HHS/United States
- R01 EY036430/EY/NEI NIH HHS/United States
- R35 NS097305/NS/NINDS NIH HHS/United States
- F32 EY033639/EY/NEI NIH HHS/United States
- U01 NS136405/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
