This is a preprint.
A Vulnerable Subtype of Dopaminergic Neurons Drives Early Motor Deficits in Parkinson's Disease
- PMID: 39763754
- PMCID: PMC11702755
- DOI: 10.1101/2024.12.20.629776
A Vulnerable Subtype of Dopaminergic Neurons Drives Early Motor Deficits in Parkinson's Disease
Abstract
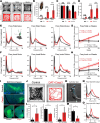
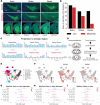
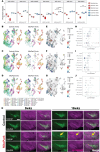
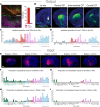
In Parkinson's disease, dopaminergic neurons (DANs) in the midbrain gradually degenerate, with ventral substantia nigra pars compacta (SNc) DANs exhibiting greater vulnerability. However, it remains unclear whether specific molecular subtypes of ventral SNc DANs are more susceptible to degeneration in PD, and if they contribute to the early motor symptoms associated with the disease. We identified a subtype of Sox6+ DANs, Anxa1+, which are selectively lost earlier than other DANs, and with a time course that aligns with the development of motor symptoms in MitoPark mice. We generated a knock-in Cre mouse line for Anxa1+ DANs and showed differential anatomical inputs and outputs of this population. Further, we found that the inhibition of transmitter release in Anxa1+ neurons led to bradykinesia and tremor. This study uncovers the existence of a selectively vulnerable subtype of DANs that is sufficient to drive early motor symptoms in Parkinson's disease.
Keywords: Parkinson’s disease; cell types; dopamine; motor symptoms; neurodegeneration; tremor; vulnerability.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Hirsch E.C., Graybiel A.M., and Agid Y. (1988). Melanized dopaminergic neurons are differentially affected in Parkinson’s disease. Nature 334, 345−−348. - PubMed
-
- HORNYKIEWICZ O. (1963). [The tropical localization and content of noradrenalin and dopamine (3-hydroxytyramine) in the substantia nigra of normal persons and patients with Parkinson’s disease]. Wien Klin Wochenschr 75, 309—312. - PubMed
-
- Poulin J.F., Gaertner Z., Moreno-Ramos O.A., and Awatramani R. (2020). Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Preprint at Elsevier Ltd, https://doi.org/10.1016/j.tins.2020.01.004 https://doi.org/10.1016/j.tins.2020.01.004. - DOI - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
