This is a preprint.
Ultrastructural membrane dynamics of mouse and human cortical synapses
- PMID: 39763840
- PMCID: PMC11703259
- DOI: 10.1101/2024.12.26.630393
Ultrastructural membrane dynamics of mouse and human cortical synapses
Update in
-
Ultrastructural membrane dynamics of mouse and human cortical synapses.Neuron. 2025 Nov 24:S0896-6273(25)00837-2. doi: 10.1016/j.neuron.2025.10.030. Online ahead of print. Neuron. 2025. PMID: 41289997 Free PMC article.
Abstract
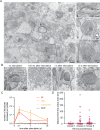
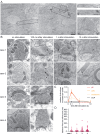
Live human brain tissues provide unique opportunities for understanding synaptic transmission. Investigations have been limited to anatomy, electrophysiology, and protein localization-while crucial parameters such as synaptic vesicle dynamics were not visualized. Here we utilize zap-and-freeze time-resolved electron microscopy to overcome this hurdle. First, we validate the approach with acute mouse brain slices to demonstrate that slices can be stimulated to produce calcium signaling. Next, we show that synaptic vesicle endocytosis is induced in both mouse and human brain slices. Crucially, clathrin-free endocytic pits appear immediately next to the active zone, where ultrafast endocytosis normally occurs, and can be trapped at this location by a dynamin inhibitor. In both species a protein essential for ultrafast endocytosis, Dynamin 1xA, localizes to the region peripheral to the active zone, the putative endocytic zone, indicating a possible conserved mechanism between mouse and human. This approach has the potential to reveal dynamic, high-resolution information about synaptic membrane trafficking in intact human brain slices.
Keywords: 2-photon calcium imaging; Dynamin 1xA; cerebellum; cortex; high-pressure freezing; human neocortex; stimulated emission depletion microscopy; synaptic transmission; synaptic vesicle endocytosis; time-resolved electron microscopy; ultrafast endocytosis; zap-and-freeze.
Figures





References
-
- Barzó P, Szöts I, Tóth M, Csajbók ÉA, Molnár G, Tamás G. Electrophysiology and Morphology of Human Cortical Supragranular Pyramidal Cells in a Wide Age Range. eLife. 2024;13. doi: 10.7554/eLife.100390.1 - DOI
-
- Hunt S, Leibner Y, Mertens EJ, et al. Strong and reliable synaptic communication between pyramidal neurons in adult human cerebral cortex. Cereb Cortex. 2022;33(6):2857–2878. doi: 10.1093/cercor/bhac246 - DOI
-
- Watson JF, Vargas-Barroso V, Morse-Mora RJ, et al. Human hippocampal CA3 uses specific functional connectivity rules for efficient associative memory. Cell. 2024;0(0). doi: 10.1016/j.cell.2024.11.022 - DOI
-
- Nahirney PC, Tremblay ME. Brain Ultrastructure: Putting the Pieces Together. Frontiers in Cell and Developmental Biology. 2021;9. Accessed January 1, 2024. https://www.frontiersin.org/articles/ 10.3389/fcell.2021.629503 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
