This is a preprint.
Dynamics of postnatal bone development and epiphyseal synostosis in the caprine autopod
- PMID: 39763848
- PMCID: PMC11703179
- DOI: 10.1101/2024.12.26.630423
Dynamics of postnatal bone development and epiphyseal synostosis in the caprine autopod
Update in
-
Dynamics of postnatal bone development and epiphyseal synostosis in the caprine autopod.Dev Dyn. 2025 May 13:10.1002/dvdy.70038. doi: 10.1002/dvdy.70038. Online ahead of print. Dev Dyn. 2025. PMID: 40359336
Abstract
Bones develop to structurally balance strength and mobility. Bone developmental dynamics are influenced by whether an animal is ambulatory at birth (i.e., precocial). Precocial species, such as goats, develop advanced skeletal maturity in utero, making them useful models for studying the dynamics of bone formation under mechanical load. Here, we used microcomputed tomography and histology to characterize postnatal bone development in the autopod of the caprine lower forelimb. The caprine autopod features two toes, fused by metacarpal synostosis (i.e., bone fusion) prior to birth. Our analysis focused on the phalanges 1 (P1) and metacarpals of the goat autopod from birth through adulthood (3.5 years). P1 cortical bone densified rapidly after birth (half-life using one-phase exponential decay model (τ1/2 = 1.6 ± 0.4 months), but the P1 cortical thickness increased continually through adulthood (τ1/2 = 7.2 ± 2.7 mo). Upon normalization by body mass, the normalized polar moment of inertia of P1 cortical bone was constant over time, suggestive of structural load adaptation. P1 trabecular bone increased in trabecular number (τ1/2 = 6.7 ± 2.8 mo) and thickness (τ1/2 = 6.6 ± 2.0 mo) until skeletal maturity, while metacarpal trabeculae grew primarily through trabecular thickening (τ1/2 = 7.9 ± 2.2 mo). Unlike prenatal fusion of the metacarpal diaphysis, synostosis of the epiphyses occurred postnatally, prior to growth plate closure, through a unique fibrocartilaginous endochondral ossification. These findings implicate ambulatory loading in postnatal bone development of precocial goats and identify a novel postnatal synostosis event in the caprine metacarpal epiphysis.
Keywords: Bone Development; Bone Fusion; Bone Morphometry; Caprine; Microcomputed Tomography.
Figures




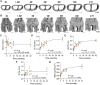

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
