This is a preprint.
Dopaminergic responses to identity prediction errors depend differently on the orbitofrontal cortex and hippocampus
- PMID: 39763911
- PMCID: PMC11702580
- DOI: 10.1101/2024.12.11.628003
Dopaminergic responses to identity prediction errors depend differently on the orbitofrontal cortex and hippocampus
Update in
-
Dopaminergic responses to identity prediction errors depend differently on the orbitofrontal cortex and hippocampus.Behav Neurosci. 2025 Sep 1. doi: 10.1037/bne0000633. Online ahead of print. Behav Neurosci. 2025. PMID: 40892558
Abstract
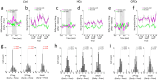
Adaptive behavior depends on the ability to predict specific events, particularly those related to rewards. Armed with such associative information, we can infer the current value of predicted rewards based on changing circumstances and desires. To support this ability, neural systems must represent both the value and identity of predicted rewards, and these representations must be updated when they change. Here we tested whether prediction error signaling of dopamine neurons depends on two areas known to represent the specifics of rewarding events, the HC and OFC. We monitored the spiking activity of dopamine neurons in rat VTA during changes in the number or flavor of expected rewards designed to induce errors in the prediction of reward value or reward identity, respectively. In control animals, dopamine neurons registered both error types, transiently increasing firing to additional drops of reward or changes in reward flavor. These canonical firing signatures of value and identity prediction errors were significantly disrupted in rats with ipsilateral neurotoxic lesions of either HC or OFC. Specifically, HC lesions caused a failure to register either type of prediction error, whereas OFC lesions caused persistent signaling of identity prediction errors and much more subtle effects on signaling of value errors. These results demonstrate that HC and OFC contribute distinct types of information to the computation of prediction errors signaled by dopaminergic neurons.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Behrens T.E., Muller T.H., Whittington J.C.R., Mark S., Baram A.B., Stachenfeld K.L., and Kurth-Nelson Z. (2018). What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509. - PubMed
-
- Chudasama Y., Kralik J.D., and Murray E.A. (2007). Rhesus monkeys with orbital prefrontal cortex lesions can learn to inhibit prepotent responses in the reversed reward contingency task. Cerebral Cortex 17, 1154–1159. - PubMed
-
- Daw N.D., Niv Y., and Dayan P. (2005). Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience 8, 1704–1711 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
