This is a preprint.
SLC35A2 loss of function variants affect glycomic signatures, neuronal fate, and network dynamics
- PMID: 39763953
- PMCID: PMC11703275
- DOI: 10.1101/2024.12.27.630524
SLC35A2 loss of function variants affect glycomic signatures, neuronal fate, and network dynamics
Update in
-
SLC35A2 loss-of-function variants affect glycomic signatures, neuronal fate and network dynamics.Brain. 2025 Dec 4;148(12):4259-4274. doi: 10.1093/brain/awaf198. Brain. 2025. PMID: 40418734
Abstract
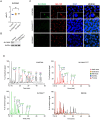
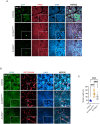
SLC35A2 encodes a UDP-galactose transporter essential for glycosylation of proteins and galactosylation of lipids and glycosaminoglycans. Germline genetic SLC35A2 variants have been identified in congenital disorders of glycosylation and somatic SLC35A2 variants have been linked to intractable epilepsy associated with malformations of cortical development. However, the functional consequences of these pathogenic variants on brain development and network integrity remain elusive. In this study, we use an isogenic human induced pluripotent stem cell-derived neuron model to comprehensively interrogate the functional impact of loss of function variants in SLC35A2 through the integration of cellular and molecular biology, protein glycosylation analysis, neural network dynamics, and single cell electrophysiology. We show that loss of function variants in SLC35A2 result in disrupted glycomic signatures and precocious neurodevelopment, yielding hypoactive, asynchronous neural networks. This aberrant network activity is attributed to an inhibitory/excitatory imbalance as characterization of neural composition revealed preferential differentiation of SLC35A2 loss of function variants towards the GABAergic fate. Additionally, electrophysiological recordings of synaptic activity reveal a shift in excitatory/inhibitory balance towards increased inhibitory drive, indicating changes occurring specifically at the pre-synaptic terminal. Our study is the first to provide mechanistic insight regarding the early development and functional connectivity of SLC35A2 loss of function variant harboring human neurons, providing important groundwork for future exploration of potential therapeutic interventions.
Keywords: epilepsy; glycosylation; human stem cells; neural network; neurodevelopment.
Conflict of interest statement
Competing interests The authors report no competing interests.
Figures





References
-
- Aoki K, Ishida N, Kawakita M. Substrate recognition by UDP-galactose and CMP-sialic acid transporters. Different sets of transmembrane helices are utilized for the specific recognition of UDP-galactose and CMP-sialic acid. J Biol Chem. Jun 15 2001;276(24):21555–61. doi: 10.1074/jbc.M101462200 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
