This is a preprint.
Beta cell dysfunction occurs independently of insulitis in type 1 diabetes pathogenesis
- PMID: 39763971
- PMCID: PMC11703223
- DOI: 10.1101/2024.12.29.630665
Beta cell dysfunction occurs independently of insulitis in type 1 diabetes pathogenesis
Abstract
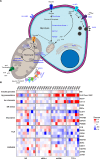
The loss of insulin secretory function associated with type 1 diabetes (T1D) is attributed to the immune-mediated destruction of beta cells. Yet, at onset of T1D, patients often have a significant beta cell mass remaining while T cell infiltration of pancreatic islets is sporadic. Thus, we investigated the hypothesis that the remaining beta cells in T1D are largely dysfunctional using live human pancreas tissue slices prepared from organ donors with recently diagnosed T1D. Beta cells in slices from donors with T1D had significantly diminished Ca2+ mobilization and insulin secretion responses to glucose. Beta cell function was equally impaired in T cell-infiltrated and non-infiltrated islets. Fixed tissue staining and gene expression profiling of laser-capture microdissected islets revealed significant decreases of proteins and genes in the glucose stimulus secretion coupling pathway. From these data, we posit that functional defects occur in the remaining mass of beta cells during human T1D pathogenesis.
Keywords: beta cell; calcium; function; gene expression; human; insulin; metabolism; pancreas; slice; type 1 diabetes.
Figures





Similar articles
-
Beta cell dysfunction occurs independently of insulitis in type 1 diabetes pathogenesis.Cell Rep. 2025 Aug 26;44(9):116174. doi: 10.1016/j.celrep.2025.116174. Online ahead of print. Cell Rep. 2025. PMID: 40875294
-
Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis.JCI Insight. 2020 Apr 23;5(8):e134525. doi: 10.1172/jci.insight.134525. JCI Insight. 2020. PMID: 32324170 Free PMC article.
-
Hyaluronan deposition in islets may precede and direct the location of islet immune-cell infiltrates.Diabetologia. 2020 Mar;63(3):549-560. doi: 10.1007/s00125-019-05066-7. Epub 2020 Jan 6. Diabetologia. 2020. PMID: 31907557 Free PMC article.
-
Deciphering the Pathogenesis of Human Type 1 Diabetes (T1D) by Interrogating T Cells from the "Scene of the Crime".Curr Diab Rep. 2017 Sep 2;17(10):95. doi: 10.1007/s11892-017-0915-y. Curr Diab Rep. 2017. PMID: 28864875 Free PMC article. Review.
-
Organ donor specimens: What can they tell us about type 1 diabetes?Pediatr Diabetes. 2015 Aug;16(5):320-30. doi: 10.1111/pedi.12286. Epub 2015 May 22. Pediatr Diabetes. 2015. PMID: 25998576 Free PMC article. Review.
References
Publication types
Grants and funding
- UC4 DK104155/DK/NIDDK NIH HHS/United States
- U01 DK135001/DK/NIDDK NIH HHS/United States
- T32 DK108736/DK/NIDDK NIH HHS/United States
- R01 DK132387/DK/NIDDK NIH HHS/United States
- R01 DK122160/DK/NIDDK NIH HHS/United States
- F31 DK130607/DK/NIDDK NIH HHS/United States
- R01 DK123292/DK/NIDDK NIH HHS/United States
- S10 OD016350/OD/NIH HHS/United States
- UH3 DK122638/DK/NIDDK NIH HHS/United States
- R01 DK124267/DK/NIDDK NIH HHS/United States
- P01 AI042288/AI/NIAID NIH HHS/United States
- R01 DK123329/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
