This is a preprint.
Chromosomal rearrangements and instability caused by the LINE-1 retrotransposon
- PMID: 39764018
- PMCID: PMC11702581
- DOI: 10.1101/2024.12.14.628481
Chromosomal rearrangements and instability caused by the LINE-1 retrotransposon
Abstract
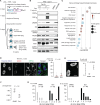
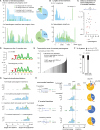
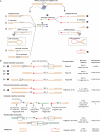
LINE-1 (L1) retrotransposition is widespread in many cancers, especially those with a high burden of chromosomal rearrangements. However, whether and to what degree L1 activity directly impacts genome integrity is unclear. Here, we apply whole-genome sequencing to experimental models of L1 expression to comprehensively define the spectrum of genomic changes caused by L1. We provide definitive evidence that L1 expression frequently and directly causes both local and long-range chromosomal rearrangements, small and large segmental copy-number alterations, and subclonal copy-number heterogeneity due to ongoing chromosomal instability. Mechanistically, all these alterations arise from DNA double-strand breaks (DSBs) generated by L1-encoded ORF2p. The processing of ORF2p-generated DSB ends prior to their ligation can produce diverse rearrangements of the target sequences. Ligation between DSB ends generated at distal loci can generate either stable chromosomes or unstable dicentric, acentric, or ring chromosomes that undergo subsequent evolution through breakage-fusion bridge cycles or DNA fragmentation. Together, these findings suggest L1 is a potent mutagenic force capable of driving genome evolution beyond simple insertions.
Figures





References
-
- Burns K. H., Repetitive DNA in disease. Science (New York, N.Y.) 376, 353–354 (2022). - PubMed
-
- Lander E. S. et al. , Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001). - PubMed
-
- Holmes S. E., Singer M. F., Swergold G. D., Studies on p40, the leucine zipper motif-containing protein encoded by the first open reading frame of an active human LINE-1 transposable element. J Biol Chem 267, 19765–19768 (1992). - PubMed
-
- Khazina E. et al. , Trimeric structure and flexibility of the L1ORF1 protein in human L1 retrotransposition. Nature structural & molecular biology 18, 1006–1014 (2011). - PubMed
-
- Weichenrieder O., Repanas K., Perrakis A., Crystal structure of the targeting endonuclease of the human LINE-1 retrotransposon. Structure 12, 975–986 (2004). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
