This is a preprint.
Targeting NLRP3 signaling with a novel sulfonylurea compound for the treatment of vascular cognitive impairment and dementia
- PMID: 39764140
- PMCID: PMC11702818
- DOI: 10.21203/rs.3.rs-5611378/v1
Targeting NLRP3 signaling with a novel sulfonylurea compound for the treatment of vascular cognitive impairment and dementia
Update in
-
Targeting NLRP3 signaling with a novel sulfonylurea compound for the treatment of vascular cognitive impairment and dementia.Fluids Barriers CNS. 2025 Jun 3;22(1):55. doi: 10.1186/s12987-025-00665-6. Fluids Barriers CNS. 2025. PMID: 40462117 Free PMC article.
Abstract
Background: As a key inflammatory factor, the nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome plays a crucial role in neuroinflammation and the progression of neurodegenerative diseases. Dysregulation of NLRP3 signaling can trigger various inflammatory responses in the brain, contributing to the development of neurodegenerative diseases such as ischemic stroke, vascular dementia (VaD), Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). Therefore, the NLRP3 signaling pathway is a promising therapeutic target for the treatment of neurodegenerative diseases, including VaD.
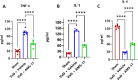
Methods: In this study, we investigated the therapeutic effects of a synthetic sulfonylurea NLRP3 inhibitor, AMS-17, in a VaD mouse model using bilateral common carotid artery stenosis (BCAS) and elucidated the underlying mechanisms. All mice were randomly divided into three groups: Sham, VaD + Vehicle, and VaD + AMS-17. Cognitive function was assessed using the Y-maze and Morris water maze (MWM) on the 50th day after BCAS. Brain sections and blood serum samples were collected for biomarker analysis and immunohistochemistry. Neurodegeneration, expressions of the molecules involved in the NLRP3 signaling pathways, tight junction proteins, and myelination were assessed using western blotting and immunofluorescence (IF). The levels of Interleukin-1 beta (IL-1β), Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-4 (IL-4) in the blood were measured using ELISA.
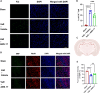
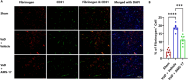
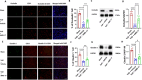
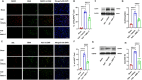
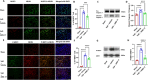
Results: AMS-17 treatment improved cognitive function, enhanced blood-brain barrier (BBB) integrity, and promoted remyelination in VaD mice. Additionally, AMS-17 reduced neurodegeneration and decreased the expression of NLRP3 and its associated proteins, Apoptosis-associated speck-like protein (ASC), and cleaved caspase-1 in the brain. It also lowered pro-inflammatory TNF-α and IL-1β levels, while increasing the anti-inflammatory IL-4 level in the blood.
Conclusions: The findings of this study provide the first promising evidence for the use of AMS-17 in VaD treatment in mice. This study introduces AMS-17 as a novel chemical scaffold with NLRP3 inhibitory activity, which can be further developed for the treatment of VaD in humans.
Keywords: Inflammasome; Inflammation; Ischemia; NLRP3; Vascular Dementia.
Figures

Similar articles
-
Targeting NLRP3 signaling with a novel sulfonylurea compound for the treatment of vascular cognitive impairment and dementia.Fluids Barriers CNS. 2025 Jun 3;22(1):55. doi: 10.1186/s12987-025-00665-6. Fluids Barriers CNS. 2025. PMID: 40462117 Free PMC article.
-
Targeting NLRP3 signaling by a novel-designed sulfonylurea compound for inhibition of microglial inflammation.Bioorg Med Chem. 2022 Mar 15;58:116645. doi: 10.1016/j.bmc.2022.116645. Epub 2022 Jan 31. Bioorg Med Chem. 2022. PMID: 35151118 Free PMC article.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
A novel strategy for bioactive natural products targeting NLRP3 inflammasome in Alzheimer's disease.Front Pharmacol. 2023 Jan 9;13:1077222. doi: 10.3389/fphar.2022.1077222. eCollection 2022. Front Pharmacol. 2023. PMID: 36699095 Free PMC article. Review.
Cited by
-
Prognostic risk modeling of endometrial cancer using programmed cell death-related genes: a comprehensive machine learning approach.Discov Oncol. 2025 Mar 8;16(1):280. doi: 10.1007/s12672-025-02039-8. Discov Oncol. 2025. PMID: 40056247 Free PMC article.
References
-
- Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer’s disease and vascular dementia. J Neurol Sci [Internet]. 2005. Mar 15 [cited 2023 Jan 30];229:43–9. Available from: https://www.jns-journal.com/article/S0022-510X(04)00445-9/fulltext - PubMed
-
- Belkhelfa M, Beder N, Mouhoub D, Amri M, Hayet R, Tighilt N, et al. The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J Neuroimmunol [Internet]. 2018. Jul 15 [cited 2023 Jan 30];320:48–57. Available from: https://www.sciencedirect.com/science/article/pii/S0165572818300316 - PubMed
-
- Dhapola R, Sarma P, Medhi B, Prakash A, Reddy DH. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Mitochondrial Dysfunction for Alzheimer’s Disease. Mol Neurobiol. 2022. Jan;59(1):535–55. - PubMed
-
- Bai R, Lang Y, Shao J, Deng Y, Refuhati R, Cui L. The Role of NLRP3 Inflammasome in Cerebrovascular Diseases Pathology and Possible Therapeutic Targets. ASN NEURO [Internet]. 2021. May 29 [cited 2024 Nov 24];13:17590914211018100. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8168029/ - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

